Abstract
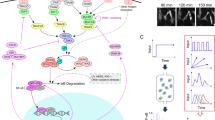
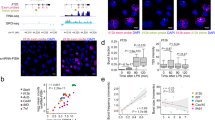
The innate immune system is like a double-edged sword: it is absolutely required for host defense against infection, but when uncontrolled, it can trigger a plethora of inflammatory diseases. Here we use systems-biology approaches to predict and confirm the existence of a gene-regulatory network involving dynamic interaction among the transcription factors NF-κB, C/EBPδ and ATF3 that controls inflammatory responses. We mathematically modeled transcriptional regulation of the genes encoding interleukin 6 and C/EBPδ and experimentally confirmed the prediction that the combination of an initiator (NF-κB), an amplifier (C/EBPδ) and an attenuator (ATF3) forms a regulatory circuit that discriminates between transient and persistent Toll-like receptor 4–induced signals. Our results suggest a mechanism that enables the innate immune system to detect the duration of infection and to respond appropriately.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Janeway, C.A. & Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 20, 197–216 (2002).
Aderem, A. & Ulevitch, R.J. Toll-like receptors in the induction of the innate immune response. Nature 406, 782–787 (2000).
Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1, 135–145 (2001).
Nathan, C. Points of control in inflammation. Nature 420, 846–852 (2002).
Kobayashi, K.S. & Flavell, R.A. Shielding the double-edged sword: negative regulation of the innate immune system. J. Leukoc. Biol. 75, 428–433 (2004).
Barnes, P.J. & Karin, M. Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 336, 1066–1071 (1997).
Bouma, G. & Strober, W. The immunological and genetic basis of inflammatory bowel disease. Nat. Rev. Immunol. 3, 521–533 (2003).
Liew, F.Y., Xu, D., Brint, E.K. & O'Neill, L.A.J. Negative regulation of toll-like receptor-mediated immune responses. Nat. Rev. Immunol. 5, 446–458 (2005).
Akira, S., Uematsu, S. & Takeuchi, O. Pathogen recognition and innate immunity. Cell 124, 783–801 (2006).
Kawai, T. & Akira, S. Pathogen recognition with Toll-like receptors. Curr. Opin. Immunol. 17, 338–344 (2005).
Royet, J., Reichhart, J.M. & Hoffmann, J.A. Sensing and signaling during infection in Drosophila. Curr. Opin. Immunol. 17, 11–17 (2005).
Takeda, K. & Akira, S. TLR signaling pathways. Semin. Immunol. 16, 3–9 (2004).
Jenner, R.G. & Young, R.A. Insights into host responses against pathogens from transcriptional profiling. Nat. Rev. Microbiol. 3, 281–294 (2005).
Beutler, B. Tlr4: Central component of the sole mammalian LPS sensor. Curr. Opin. Immunol. 12, 20–26 (2000).
Taylor, P.R. et al. Macrophage receptors and immune recognition. Annu. Rev. Immunol. 23, 901–944 (2005).
Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 3, 23–35 (2003).
Boldrick, J.C. et al. Stereotyped and specific gene expression programs in human innate immune responses to bacteria. Proc. Natl. Acad. Sci. USA 99, 972–977 (2002).
Nau, G.J. et al. Human macrophage activation programs induced by bacterial pathogens. Proc. Natl. Acad. Sci. USA 99, 1503–1508 (2002).
Roach, J.C. et al. Transcription factor expression in lipopolysaccharide-activated peripheral-blood-derived mononuclear cells. Proc. Natl. Acad. Sci. USA 104, 16245–16250 (2007).
Aderem, A. Systems biology: its practice and challenges. Cell 121, 511–513 (2005).
Kitano, H. Computational systems biology. Nature 420, 206–210 (2002).
Suthram, S., Sittler, T. & Ideker, T. The plasmodium protein network diverges from those of other eukaryotes. Nature 438, 108–112 (2005).
Ideker, T., Galitski, T. & Hood, L. A new approach to decoding life: Systems biology. Annu. Rev. Genomics Hum. Genet. 2, 343–372 (2001).
Aderem, A. & Smith, K.D. A systems approach to dissecting immunity and inflammation. Semin. Immunol. 16, 55–67 (2004).
Bolouri, H. & Davidson, E.H. Transcriptional regulatory cascades in development: initial rates, not steady state, determine network kinetics. Proc. Natl. Acad. Sci. USA 100, 9371–9376 (2003).
Smith, J., Theodoris, C. & Davidson, E.H. A gene regulatory network subcircuit drives a dynamic pattern of gene expression. Science 318, 794–797 (2007).
Gilchrist, M. et al. Systems biology approaches identify ATF3 as a negative regulator of Toll-like receptor 4. Nature 441, 173–178 (2006).
Hoffmann, A., Levchenko, A., Scott, M.L. & Baltimore, D. The IκB-NF-κB signaling module: temporal control and selective gene activation. Science 298, 1241–1245 (2002).
Ramsey, S.A. et al. Dual feedback loops in the GAL regulon suppress cellular heterogeneity in yeast. Nat. Genet. 38, 1082–1087 (2006).
Alon, U. Network motifs: theory and experimental approaches. Nat. Rev. Genet. 8, 450–461 (2007).
Flo, T.H. et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432, 917–921 (2004).
Ramsey, S.A. et al. Uncovering a macrophage transcriptional program by integrating evidence from motif scanning and expression dynamics. PLOS Comput. Biol. 4, e1000021 (2008).
Li, Q. & Verma, I.M. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2, 725–734 (2002).
Lekstrom-Himes, J. & Xanthopoulos, K.G. Biological role of the CCAAT/enhancer-binding protein family of transcription factors. J. Biol. Chem. 273, 28545–28548 (1998).
Kovács, K.A., Steinmann, M., Magistretti, P.J., Halfon, O. & Cardinaux, J.R. CCAAT/enhancer-binding protein family members recruit the coactivator CREB-binding protein and trigger its phosphorylation. J. Biol. Chem. 278, 36959–36965 (2003).
Johnson, W.E. et al. Model-based analysis of tiling-arrays for ChIP-chip. Proc. Natl. Acad. Sci. USA 103, 12457–12462 (2006).
Longabaugh, W.J.R., Davidson, E.H. & Bolouri, H. Computational representation of developmental genetic regulatory networks. Dev. Biol. 283, 1–16 (2005).
Acknowledgements
We thank M. Gilchrist, E. Gold and C. Rosenberger for discussions and critical reading of the manuscript; and A. Nachman, I. Podolsky, C. Lorang and T. Stolyar for technical assistance. Supported by Irvington Institute Fellowship Program of the Cancer Research Institute (V.L.) and the National Institutes of Health (A.A.).
Author information
Authors and Affiliations
Contributions
V.L. designed experiments, did all experimental studies and drafted the manuscript; S.A.R. did data analysis and mathematical modeling; A.G.R., D.E.Z. and M.N. did computational analysis; K.A.K. did microarray experiments; A.E.L. provided technical assistance for experiments, including quantitative real-time PCR, ChIP and in vivo studies; I.S. supervised the computational analysis; and A.A. supervised the study and wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5, Supplementary Tables 1–5 and Supplementary Methods (PDF 2004 kb)
Rights and permissions
About this article
Cite this article
Litvak, V., Ramsey, S., Rust, A. et al. Function of C/EBPδ in a regulatory circuit that discriminates between transient and persistent TLR4-induced signals. Nat Immunol 10, 437–443 (2009). https://doi.org/10.1038/ni.1721
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1721
This article is cited by
-
The linkage of NF-κB signaling pathway-associated long non-coding RNAs with tumor microenvironment and prognosis in cervical cancer
BMC Medical Genomics (2023)
-
Incomplete Knockdown of MyD88 Inhibits LPS-Induced Lung Injury and Lung Fibrosis in a Mouse Model
Inflammation (2023)
-
Translocator protein is a marker of activated microglia in rodent models but not human neurodegenerative diseases
Nature Communications (2023)
-
Perivascular tenascin C triggers sequential activation of macrophages and endothelial cells to generate a pro-metastatic vascular niche in the lungs
Nature Cancer (2022)
-
PERK signaling through C/EBPδ contributes to ER stress-induced expression of immunomodulatory and tumor promoting chemokines by cancer cells
Cell Death & Disease (2021)