Abstract
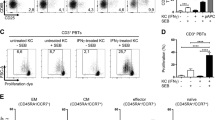
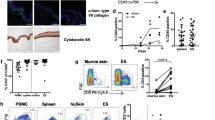
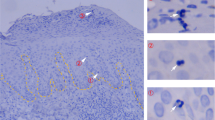
Interleukin 22 (IL-22) is a cytokine produced by the TH-17 lineage of helper T cells and NK-22 subset of natural killer cells that acts on epithelial cells and keratinocytes and has been linked to skin homeostasis and inflammation. Here we characterize a population of human skin-homing memory CD4+ T cells that expressed the chemokine receptors CCR10, CCR6 and CCR4 and produced IL-22 but neither IL-17 nor interferon-γ (IFN-γ). Clones isolated from this population produced IL-22 only and had low or undetectable expression of the TH-17 and T helper type 1 (TH1) transcription factors RORγt and T-bet. The differentiation of T cells producing only IL-22 was efficiently induced in naive T cells by plasmacytoid dendritic cells in an IL-6- and tumor necrosis factor–dependent way. Our findings delineate a previously unknown subset of human CD4+ effector T cells dedicated to skin pathophysiology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bromley, S.K., Mempel, T.R. & Luster, A.D. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat. Immunol. 9, 970–980 (2008).
Szabo, S.J. et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100, 655–669 (2000).
Zheng, W. & Flavell, R.A. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 89, 587–596 (1997).
Harrington, L.E. et al. Interleukin 17–producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6, 1123–1132 (2005).
Park, H. et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6, 1133–1141 (2005).
Liang, S.C. et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203, 2271–2279 (2006).
Veldhoen, M., Hocking, R.J., Atkins, C.J., Locksley, R.M. & Stockinger, B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189 (2006).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
Mangan, P.R. et al. Transforming growth factor-β induces development of the TH17 lineage. Nature 441, 231–234 (2006).
Acosta-Rodriguez, E.V., Napolitani, G., Lanzavecchia, A. & Sallusto, F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17–producing human T helper cells. Nat. Immunol. 8, 942–949 (2007).
Ivanov, I.I. et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006).
Yang, X.O. et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors RORα and RORγ. Immunity 28, 29–39 (2008).
Veldhoen, M. et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453, 106–109 (2008).
Langrish, C.L. et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201, 233–240 (2005).
Korn, T. et al. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature 448, 484–487 (2007).
Yang, L. et al. IL-21 and TGF-β are required for differentiation of human TH17 cells. Nature 454, 350–352 (2008).
Wilson, N.J. et al. Development, cytokine profile and function of human interleukin 17–producing helper T cells. Nat. Immunol. 8, 950–957 (2007).
Zhou, L. et al. TGF-β-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nature 453, 236–240 (2008).
Annunziato, F. et al. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 204, 1849–1861 (2007).
Butcher, E.C. & Picker, L.J. Lymphocyte homing and homeostasis. Science 272, 60–66 (1996).
Kupper, T.S. & Fuhlbrigge, R.C. Immune surveillance in the skin: mechanisms and clinical consequences. Nat. Rev. Immunol. 4, 211–222 (2004).
Salmi, M. & Jalkanen, S. Lymphocyte homing to the gut: attraction, adhesion, and commitment. Immunol. Rev. 206, 100–113 (2005).
Sallusto, F. & Mackay, C.R. Chemoattractants and their receptors in homeostasis and inflammation. Curr. Opin. Immunol. 16, 724–731 (2004).
Acosta-Rodriguez, E.V. et al. Surface phenotype and antigenic specificity of human interleukin 17–producing T helper memory cells. Nat. Immunol. 8, 639–646 (2007).
Singh, S.P., Zhang, H.H., Foley, J.F., Hedrick, M.N. & Farber, J.M. Human T cells that are able to produce IL-17 express the chemokine receptor CCR6. J. Immunol. 180, 214–221 (2008).
Reboldi, A. et al. C–C chemokine receptor 6–regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat. Immunol. 10, 514–523 (2009).
Sallusto, F., Lenig, D., Mackay, C.R. & Lanzavecchia, A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J. Exp. Med. 187, 875–883 (1998).
Lord, G.M. et al. T-bet is required for optimal proinflammatory CD4+ T-cell trafficking. Blood 106, 3432–3439 (2005).
Sigmundsdottir, H. & Butcher, E.C. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nat. Immunol. 9, 981–987 (2008).
Sigmundsdottir, H. et al. DCs metabolize sunlight-induced vitamin D3 to 'program' T cell attraction to the epidermal chemokine CCL27. Nat. Immunol. 8, 285–293 (2007).
Iwata, M. et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity 21, 527–538 (2004).
Cella, M. et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 457, 722–725 (2009).
Zenewicz, L.A. et al. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity 29, 947–957 (2008).
Luci, C. et al. Influence of the transcription factor RORγt on the development of NKp46+ cell populations in gut and skin. Nat. Immunol. 10, 75–82 (2009).
Wolk, K. & Sabat, R. Interleukin-22: a novel T- and NK-cell derived cytokine that regulates the biology of tissue cells. Cytokine Growth Factor Rev. 17, 367–380 (2006).
Wolk, K. et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur. J. Immunol. 36, 1309–1323 (2006).
McGeachy, M.J. et al. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell–mediated pathology. Nat. Immunol. 8, 1390–1397 (2007).
Zheng, Y. et al. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 445, 648–651 (2007).
Ma, H.L. et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J. Clin. Invest. 118, 597–607 (2008).
Boniface, K. et al. A role for T cell-derived interleukin 22 in psoriatic skin inflammation. Clin. Exp. Immunol. 150, 407–415 (2007).
Reiss, Y., Proudfoot, A.E., Power, C.A., Campbell, J.J. & Butcher, E.C. CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J. Exp. Med. 194, 1541–1547 (2001).
Seder, R.A., Darrah, P.A. & Roederer, M. T-cell quality in memory and protection: implications for vaccine design. Nat. Rev. Immunol. 8, 247–258 (2008).
Hughes, T. et al. Stage three immature human natural killer cells found in secondary lymphoid tissue constitutively and selectively express the TH17 cytokine interleukin-22. Blood 113, 4008–4010 (2009).
Wolk, K. et al. IL-22 increases the innate immunity of tissues. Immunity 21, 241–254 (2004).
Messi, M. et al. Memory and flexibility of cytokine gene expression as separable properties of human TH1 and TH2 lymphocytes. Nat. Immunol. 4, 78–86 (2003).
Rivino, L. et al. Chemokine receptor expression identifies Pre-T helper (Th)1, Pre-Th2, and nonpolarized cells among human CD4+ central memory T cells. J. Exp. Med. 200, 725–735 (2004).
Trifari, S., Kaplan, C.D., Tran, E.H., Crellin, N.K. & Spitz, H. Identification of a human helper T cell population that produces abundant IL-22 and is distinct from TH-17, TH1 and TH2 cells. Nat. Immunol. advance online publication, doi:10.1038/ni1770 (5 July 2009).
Homey, B. et al. CCL27–CCR10 interactions regulate T cell-mediated skin inflammation. Nat. Med. 8, 157–165 (2002).
Gilliet, M., Cao, W. & Liu, Y.J. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat. Rev. Immunol. 8, 594–606 (2008).
Albanesi, C. et al. Chemerin expression marks early psoriatic skin lesions and correlates with plasmacytoid dendritic cell recruitment. J. Exp. Med. 206, 249–258 (2009).
Lande, R. et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 449, 564–569 (2007).
Nograles, K.E. et al. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J. Allergy Clin. Immunol. 123, 1244–1252 (2009).
Acknowledgements
We thank G. Napolitani for discussions; A. Cavani (Istituto Dermopatico dell'Immacolata) for T cells from psoriatic skin lesions; and M. Roederer (US National Institutes of Health) for the Spice and Pestle software. Supported by the Swiss National Science Foundation (31-101962 to F.S.), the European Commission Framework Programme 6 (Innovative Chemokine-based Therapeutic Strategies for Autoimmunity and Chronic Inflammation; LSB-CT-2005-518167) and the Helmut Horten Foundation (to the Institute for Research in Biomedicine).
Author information
Authors and Affiliations
Contributions
T.D. designed and did experiments and analyzed data; R.G. did experiments and analyzed data; D.J. sorted T cell populations; A.L. wrote the manuscript; and F.S. designed experiments, analyzed data, wrote the manuscript and provided overall direction.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 (PDF 1314 kb)
Rights and permissions
About this article
Cite this article
Duhen, T., Geiger, R., Jarrossay, D. et al. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol 10, 857–863 (2009). https://doi.org/10.1038/ni.1767
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1767
This article is cited by
-
Th22 is the effector cell of thymosin β15-induced hair regeneration in mice
Inflammation and Regeneration (2024)
-
T-cell surveillance of the human brain in health and multiple sclerosis
Seminars in Immunopathology (2022)
-
Interleukin-22 regulates neutrophil recruitment in ulcerative colitis and is associated with resistance to ustekinumab therapy
Nature Communications (2022)
-
Major depression favors the expansion of Th17-like cells and decrease the proportion of CD39+Treg cell subsets in response to myelin antigen in multiple sclerosis patients
Cellular and Molecular Life Sciences (2022)
-
The NOTCH-HES-1 axis is involved in promoting Th22 cell differentiation
Cellular & Molecular Biology Letters (2021)