Abstract
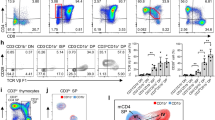
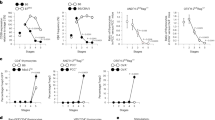
During positive selection, thymocytes transition through a stage during which T cell antigen receptor (TCR) signaling controls CD4-versus-CD8 lineage 'choice' and subsequent maturation. Here we describe a previously unknown T cell–specific protein, Themis, that serves a distinct function during this stage. In Themis−/− mice, thymocyte selection was impaired and the number of transitional CD4+CD8int thymocytes as well as CD4+ or CD8+ single-positive thymocytes was lower. Notably, although we detected no overt TCR-proximal signaling deficiencies, Themis−/− CD4+CD8int thymocytes showed developmental defects consistent with attenuated signaling that were reversible by TCR stimulation. Our results identify Themis as a critical component of the T cell developmental program and suggest that Themis functions to sustain and/or integrate signals required for proper lineage commitment and maturation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Change history
19 August 2009
NOTE: In the version of this article initially published, citation of the linked articles published in the same issue (ni.1769 and ni.1766) is missing. These should be cited on page 841 at the end of the first paragraph of the Results section. The error has been corrected in the HTML and PDF versions of the article.
References
Singer, A., Adoro, S. & Park, J.H. Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice. Nat. Rev. Immunol. 8, 788–801 (2008).
Brugnera, E. et al. Coreceptor reversal in the thymus: signaled CD4+8+ thymocytes initially terminate CD8 transcription even when differentiating into CD8+ T cells. Immunity 13, 59–71 (2000).
Bosselut, R. CD4/CD8-lineage differentiation in the thymus: from nuclear effectors to membrane signals. Nat. Rev. Immunol. 4, 529–540 (2004).
Aliahmad, P. & Kaye, J. Commitment issues: linking positive selection signals and lineage diversification in the thymus. Immunol. Rev. 209, 253–273 (2006).
Kappes, D.J., He, X. & He, X. Role of the transcription factor Th-POK in CD4:CD8 lineage commitment. Immunol. Rev. 209, 237–252 (2006).
Hernandez-Hoyos, G., Anderson, M.K., Wang, C., Rothenberg, E.V. & Alberola-Ila, J. GATA-3 expression is controlled by TCR signals and regulates CD4/CD8 differentiation. Immunity 19, 83–94 (2003).
Wang, L. et al. Distinct functions for the transcription factors GATA-3 and ThPOK during intrathymic differentiation of CD4+ T cells. Nat. Immunol. 9, 1122–1130 (2008).
Mayer, B.J. & Eck, M.J. SH3 domains. Minding your p's and q's. Curr. Biol. 5, 364–367 (1995).
Huang, Y.H., Li, D., Winoto, A. & Robey, E.A. Distinct transcriptional programs in thymocytes responding to T cell receptor, Notch, and positive selection signals. Proc. Natl. Acad. Sci. USA 101, 4936–4941 (2004).
Mick, V.E., Starr, T.K., McCaughtry, T.M., McNeil, L.K. & Hogquist, K.A. The regulated expression of a diverse set of genes during thymocyte positive selection in vivo. J. Immunol. 173, 5434–5444 (2004).
Takahama, Y., Shores, E.W. & Singer, A. Negative selection of precursor thymocytes before their differentiation into CD4+CD8+ cells. Science 258, 653–656 (1992).
Lucas, B. & Germain, R.N. Unexpectedly complex regulation of CD4/CD8 coreceptor expression supports a revised model for CD4+CD8+ thymocyte differentiation. Immunity 5, 461–477 (1996).
Lundberg, K., Heath, W., Kontgen, F., Carbone, F.R. & Shortman, K. Intermediate steps in positive selection: differentiation of CD4+8intTCRint thymocytes into CD4−8+TCRhi thymocytes. J. Exp. Med. 181, 1643–1651 (1995).
Suzuki, H., Punt, J.A., Granger, L.G. & Singer, A. Asymmetric signaling requirements for thymocyte commitment to the CD4+ versus CD8+ T cell lineages: a new perspective on thymic commitment and selection. Immunity 2, 413–425 (1995).
Cibotti, R., Punt, J.A., Dash, K.S., Sharrow, S.O. & Singer, A. Surface molecules that drive T cell development in vitro in the absence of thymic epithelium and in the absence of lineage-specific signals. Immunity 6, 245–255 (1997).
Park, J.H. et al. 'Coreceptor tuning': cytokine signals transcriptionally tailor CD8 coreceptor expression to the self-specificity of the TCR. Nat. Immunol. 8, 1049–1059 (2007).
Hare, K.J., Jenkinson, E.J. & Anderson, G. An essential role for the IL-7 receptor during intrathymic expansion of the positively selected neonatal T cell repertoire. J. Immunol. 165, 2410–2414 (2000).
Yasutomo, K., Doyle, C., Miele, L., Fuchs, C. & Germain, R.N. The duration of antigen receptor signalling determines CD4+ versus CD8+ T-cell lineage fate. Nature 404, 506–510 (2000).
Itano, A. et al. The cytoplasmic domain of CD4 promotes the development of CD4 lineage T cells. J. Exp. Med. 183, 731–741 (1996).
Liu, X. & Bosselut, R. Duration of TCR signaling controls CD4–CD8 lineage differentiation in vivo. Nat. Immunol. 5, 280–288 (2004).
Wilkinson, B. et al. TOX: an HMG box protein implicated in the regulation of thymocyte selection. Nat. Immunol. 3, 272–280 (2002).
Shao, H., Kono, D.H., Chen, L.Y., Rubin, E.M. & Kaye, J. Induction of the early growth response (Egr) family of transcription factors during thymic selection. J. Exp. Med. 185, 731–744 (1997).
Pai, S.Y. et al. Critical roles for transcription factor GATA-3 in thymocyte development. Immunity 19, 863–875 (2003).
He, X. et al. The zinc finger transcription factor Th-POK regulates CD4 versus CD8 T-cell lineage commitment. Nature 433, 826–833 (2005).
He, X. et al. CD4–CD8 lineage commitment is regulated by a silencer element at the ThPOK transcription-factor locus. Immunity 28, 346–358 (2008).
Nasreen, M., Ueno, T., Saito, F. & Takahama, Y. In vivo treatment of class II MHC-deficient mice with anti-TCR antibody restores the generation of circulating CD4 T cells and optimal architecture of thymic medulla. J. Immunol. 171, 3394–3400 (2003).
Maurice, D., Hooper, J., Lang, G. & Weston, K. c-Myb regulates lineage choice in developing thymocytes via its target gene Gata3. EMBO J. 26, 3629–3640 (2007).
Egawa, T. & Littman, D.R. ThPOK acts late in specification of the helper T cell lineage and suppresses Runx-mediated commitment to the cytotoxic T cell lineage. Nat. Immunol. 9, 1131–1139 (2008).
Yu, Q., Erman, B., Bhandoola, A., Sharrow, S.O. & Singer, A. In vitro evidence that cytokine receptor signals are required for differentiation of double positive thymocytes into functionally mature CD8+ T cells. J. Exp. Med. 197, 475–487 (2003).
Veis, D.J., Sentman, C.L., Bach, E.A. & Korsmeyer, S.J. Expression of the Bcl-2 protein in murine and human thymocytes and in peripheral T lymphocytes. J. Immunol. 151, 2546–2554 (1993).
Gong, Q. et al. Disruption of T cell signaling networks and development by Grb2 haploid insufficiency. Nat. Immunol. 2, 29–36 (2001).
Aliahmad, P. & Kaye, J. Development of all CD4 T lineages requires nuclear factor TOX. J. Exp. Med. 205, 245–256 (2008).
Zhang, W. et al. Essential role of LAT in T cell development. Immunity 10, 323–332 (1999).
Shores, E.W. et al. Role of TCR ζ chain in T cell development and selection. Science 266, 1047–1050 (1994).
Sun, G. et al. The zinc finger protein cKrox directs CD4 lineage differentiation during intrathymic T cell positive selection. Nat. Immunol. 6, 373–381 (2005).
Sommers, C.L. et al. A LAT mutation that inhibits T cell development yet induces lymphoproliferation. Science 296, 2040–2043 (2002).
Cheadle, C., Vawter, M.P., Freed, W.J. & Becker, K.G. Analysis of microarray data using Z score transformation. J. Mol. Diagn. 5, 73–81 (2003).
Johnson, A.L. et al. Themis is a member of a new metazoan gene family and is required for the completion of thymocyte positive selection. Nat. Immunol. 10, 831–839 (2009).
Fu, G. et al. Themis controls thymocyte selection through regulation of T cell antigen receptor–mediated signaling. Nat. Immunol. 10, 848–856 (2009).
Acknowledgements
We thank A. Grinberg for assistance with gene-targeting experiments and the generation of chimeric mice; A. Singer (National Cancer Institute) for Bcl-2-transgenic and Cd8a−/− mice and anti-TCRβ; J. Kaye (The Scripps Research Institute) for rabbit polyclonal anti-Tox; D. Littman (New York University) for antibody to Runx1 and Runx3; D. El-Khoury for technical assistance; and A. Singer, L. Samelson, B.J. Fowlkes and S. Hayes for critical reading of the manuscript. Supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Cancer Institute, National Institute on Aging, Center for Cancer Research, US National Institutes of Health.
Author information
Authors and Affiliations
Contributions
R.L., S.U. and P.E.L. designed research; R.L., S.U, J.L, K.-D.S., L.L. and J.P. did research; Y.Z. and N.-P.W. did and analyzed microarray experiments; K.F.W., L.W. and R.B. generated GATA-3-transgenic mice and antibodies to ThPOK; and R.L and P.E.L. wrote the paper.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–14 (PDF 2261 kb)
Rights and permissions
About this article
Cite this article
Lesourne, R., Uehara, S., Lee, J. et al. Themis, a T cell–specific protein important for late thymocyte development. Nat Immunol 10, 840–847 (2009). https://doi.org/10.1038/ni.1768
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1768
This article is cited by
-
Themis suppresses the effector function of CD8+ T cells in acute viral infection
Cellular & Molecular Immunology (2023)
-
Themis regulates metabolic signaling and effector functions in CD4+ T cells by controlling NFAT nuclear translocation
Cellular & Molecular Immunology (2021)
-
T cell receptor and cytokine signal integration in CD8+ T cells is mediated by the protein Themis
Nature Immunology (2020)
-
T cell receptor signaling for γδT cell development
Inflammation and Regeneration (2019)
-
Syndromic immune disorder caused by a viable hypomorphic allele of spliceosome component Snrnp40
Nature Immunology (2019)