Abstract
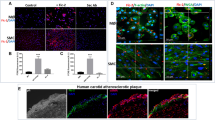
Atherosclerotic plaque formation is fueled by the persistence of lipid-laden macrophages in the artery wall. The mechanisms by which these cells become trapped, thereby establishing chronic inflammation, remain unknown. Here we found that netrin-1, a neuroimmune guidance cue, was secreted by macrophages in human and mouse atheroma, where it inactivated the migration of macrophages toward chemokines linked to their egress from plaques. Acting via its receptor, UNC5b, netrin-1 inhibited the migration of macrophages directed by the chemokines CCL2 and CCL19, activation of the actin-remodeling GTPase Rac1 and actin polymerization. Targeted deletion of netrin-1 in macrophages resulted in much less atherosclerosis in mice deficient in the receptor for low-density lipoprotein and promoted the emigration of macrophages from plaques. Thus, netrin-1 promoted atherosclerosis by retaining macrophages in the artery wall. Our results establish a causative role for negative regulators of leukocyte migration in chronic inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Moore, K.J. & Tabas, I. Macrophage in the pathogenesis of atherosclerosis. Cell 145, 341–355 (2011).
Libby, P. & Aikawa, M. Stabilization of atherosclerotic plaques: new mechanisms and clinical targets. Nat. Med. 8, 1257–1262 (2002).
Bellingan, G.J., Caldwell, H., Howie, S.E., Dransfield, I. & Haslett, C. In vivo fate of the inflammatory macrophage during the resolution of inflammation: inflammatory macrophages do not die locally, but emigrate to the draining lymph nodes. J. Immunol. 157, 2577–2585 (1996).
Randolph, G.J. Emigration of monocyte-derived cells to lymph nodes during resolution of inflammation and its failure in atherosclerosis. Curr. Opin. Lipidol. 19, 462–468 (2008).
Rong, J.X. et al. Elevating high-density lipoprotein cholesterol in apolipoprotein E-deficient mice remodels advanced atherosclerotic lesions by decreasing macrophage and increasing smooth muscle cell content. Circulation 104, 2447–2452 (2001).
Llodrá, J. et al. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc. Natl. Acad. Sci. USA 101, 11779–11784 (2004).
Feig, J.E. et al. Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques. Circulation 123, 989–998 (2011).
Feig, J.E. et al. LXR promotes the maximal egress of monocyte-derived cells from mouse aortic plaques during atherosclerosis regression. J. Clin. Invest. 120, 4415–4424 (2010).
Trogan, E. et al. Gene expression changes in foam cells and the role of chemokine receptor CCR7 during atherosclerosis regression in ApoE-deficient mice. Proc. Natl. Acad. Sci. USA 103, 3781–3786 (2006).
Cirulli, V. & Yebra, M. Netrins: beyond the brain. Nat. Rev. Mol. Cell Biol. 8, 296–306 (2007).
Keleman, K. & Dickson, B.J. Short- and long-range repulsion by the Drosophila Unc5 netrin receptor. Neuron 32, 605–617 (2001).
Srinivasan, K., Strickland, P., Valdes, A., Shin, G.C. & Hinck, L. Netrin-1/neogenin interaction stabilizes multipotent progenitor cap cells during mammary gland morphogenesis. Dev. Cell 4, 371–382 (2003).
Salminen, M., Meyer, B.I., Bober, E. & Gruss, P. Netrin 1 is required for semicircular canal formation in the mouse inner ear. Development 127, 13–22 (2000).
Nguyen, A. & Cai, H. Netrin-1 induces angiogenesis via a DCC-dependent ERK1/2-eNOS feed-forward mechanism. Proc. Natl. Acad. Sci. USA 103, 6530–6535 (2006).
Wilson, B.D. et al. Netrins promote developmental and therapeutic angiogenesis. Science 313, 640–644 (2006).
Arakawa, H. Netrin-1 and its receptors in tumorigenesis. Nat. Rev. Cancer 4, 978–987 (2004).
Fitamant, J. et al. Netrin-1 expression confers a selective advantage for tumor cell survival in metastatic breast cancer. Proc. Natl. Acad. Sci. USA 105, 4850–4855 (2008).
Ly, N.P. et al. Netrin-1 inhibits leukocyte migration in vitro and in vivo. Proc. Natl. Acad. Sci. USA 102, 14729–14734 (2005).
Mirakaj, V. et al. Netrin-1 dampens pulmonary inflammation during acute lung injury. Am. J. Respir. Crit. Care Med. 181, 815–824 (2010).
Rosenberger, P. et al. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat. Immunol. 10, 195–202 (2009).
Wang, W., Reeves, W.B. & Ramesh, G. Netrin-1 and kidney injury. I. Netrin-1 protects against ischemia-reperfusion injury of the kidney. Am. J. Physiol. Renal Physiol. 294, F739–F747 (2008).
Park, Y.M., Febbraio, M. & Silverstein, R.L. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J. Clin. Invest. 119, 136–145 (2009).
Janabi, M. et al. Oxidized LDL-induced NF-kappa B activation and subsequent expression of proinflammatory genes are defective in monocyte-derived macrophages from CD36-deficient patients. Arterioscler. Thromb. Vasc. Biol. 20, 1953–1960 (2000).
Stewart, C.R. et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 11, 155–161 (2010).
Bellingan, G.J. et al. Adhesion molecule-dependent mechanisms regulate the rate of macrophage clearance during the resolution of peritoneal inflammation. J. Exp. Med. 196, 1515–1521 (2002).
Tacke, F. et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. 117, 185–194 (2007).
Sato, N. et al. CC chemokine receptor (CCR)2 is required for langerhans cell migration and localization of T helper cell type 1 (Th1)-inducing dendritic cells. Absence of CCR2 shifts the Leishmania major-resistant phenotype to a susceptible state dominated by Th2 cytokines, b cell outgrowth, and sustained neutrophilic inflammation. J. Exp. Med. 192, 205–218 (2000).
Jimenez, F. et al. CCR2 plays a critical role in dendritic cell maturation: possible role of CCL2 and NF-kappa B. J. Immunol. 184, 5571–5581 (2010).
Delaire, S. et al. Biological activity of soluble CD100. II. Soluble CD100, similarly to H-SemaIII, inhibits immune cell migration. J. Immunol. 166, 4348–4354 (2001).
Muñoz, J.J. et al. Expression and function of the Eph A receptors and their ligands ephrins A in the rat thymus. J. Immunol. 169, 177–184 (2002).
Wu, J.Y. et al. The neuronal repellent Slit inhibits leukocyte chemotaxis induced by chemotactic factors. Nature 410, 948–952 (2001).
Wang, W., Reeves, W.B., Pays, L., Mehlen, P. & Ramesh, G. Netrin-1 overexpression protects kidney from ischemia reperfusion injury by suppressing apoptosis. Am. J. Pathol. 175, 1010–1018 (2009).
Khan, J.A. et al. Systemic human Netrin-1 gene delivery by adeno-associated virus type 8 alters leukocyte accumulation and atherogenesis in vivo. Gene Ther. (2010).
Paradisi, A. et al. NF-kappaB regulates netrin-1 expression and affects the conditional tumor suppressive activity of the netrin-1 receptors. Gastroenterology 135, 1248–1257 (2008).
Parathath, S. et al. Hypoxia is present in murine atherosclerotic plaques and has multiple adverse effects on macrophage lipid metabolism. Circ. Res. 109, 1141–1152 (2011).
Curtiss, L.K. Reversing atherosclerosis? N. Engl. J. Med. 360, 1144–1146 (2009).
Gerrity, R.G. The role of the monocyte in atherogenesis: II. Migration of foam cells from atherosclerotic lesions. Am. J. Pathol. 103, 191–200 (1981).
Kling, D., Holzschuh, T. & Betz, E. Recruitment and dynamics of leukocytes in the formation of arterial intimal thickening–a comparative study with normo- and hypercholesterolemic rabbits. Atherosclerosis 101, 79–96 (1993).
Landers, S.C., Gupta, M. & Lewis, J.C. Ultrastructural localization of tissue factor on monocyte-derived macrophages and macrophage foam cells associated with atherosclerotic lesions. Virchows Arch. 425, 49–54 (1994).
Williams, K.J., Feig, J.E. & Fisher, E.A. Rapid regression of atherosclerosis: insights from the clinical and experimental literature. Nat. Clin. Pract. Cardiovasc. Med. 5, 91–102 (2008).
Moore, K.J. et al. A CD36-initiated signaling cascade mediates inflammatory effects of β-amyloid. J. Biol. Chem. 277, 47373–47379 (2002).
Kunjathoor, V.V. et al. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J. Biol. Chem. 277, 49982–49988 (2002).
Cao, C., Lawrence, D.A., Strickland, D.K. & Zhang, L. A specific role of integrin Mac-1 in accelerated macrophage efflux to the lymphatics. Blood 106, 3234–3241 (2005).
Moore, K.J. et al. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J. Clin. Invest. 115, 2192–2201 (2005).
Manning-Tobin, J.J. et al. Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arterioscler. Thromb. Vasc. Biol. 29, 19–26 (2009).
Acknowledgements
We thank J. Goss and W. Groot for technical support; M. Tessier-Lavigne (Stanford University) for Ntn1+/− mice; and T. Kinane (Massachusetts General Hospital) for anti-UNC5b antiserum. Supported by the American Heart Association (0655840T to K.M. and 09POST2080250 to J.M.v.G.), the US National Institutes of Health (RC1HL100815 to K.J.M. and R01HL084312 to E.A.F.), the Heart and Stroke Foundation of Canada (K.J.R.) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (01558/2007-6 to L.R.F. and J.I.A.-L.).
Author information
Authors and Affiliations
Contributions
J.M.v.G. did migration and atherosclerosis studies; M.C.D. did smooth muscle studies and fetal liver cell transplantation; K.J.R. and L.R.F. did mouse atherosclerosis studies, J.I.A.-L., S.P. and B.R. did microscopy; T.D.R., A.J.R. and J.L.F. did biochemical assays; T.O.M. and K.D.O. did immunohistochemical studies of human atheroma; E.D. and E.A.F. assisted in bead-labeling experiments, L.M.S. and A.L.-H. contributed to experimental design, data analysis and provided discussions; K.J.M. designed, analyzed and interpreted the studies and wrote the manuscript with J.M.v.G.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 and Tables 1–2 (PDF 3018 kb)
Rights and permissions
About this article
Cite this article
van Gils, J., Derby, M., Fernandes, L. et al. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat Immunol 13, 136–143 (2012). https://doi.org/10.1038/ni.2205
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2205
This article is cited by
-
Macrophage profiling in atherosclerosis: understanding the unstable plaque
Basic Research in Cardiology (2024)
-
Phytol from Scoparia dulcis prevents NF-κB-mediated inflammatory responses during macrophage polarization
3 Biotech (2024)
-
Hypofucosylation of Unc5b regulated by Fut8 enhances macrophage emigration and prevents atherosclerosis
Cell & Bioscience (2023)
-
A Lipid-Structured Model of Atherosclerotic Plaque Macrophages with Lipid-Dependent Kinetics
Bulletin of Mathematical Biology (2023)
-
Netrin-1 and RGMa: Novel Regulators of Atherosclerosis-Related Diseases
Cardiovascular Drugs and Therapy (2023)