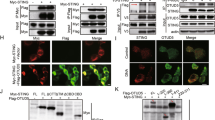
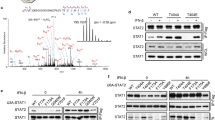
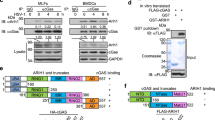
Abstract
The sensing of viral nucleic acids by the innate immune system triggers the production of type I interferons, which activates interferon-stimulated genes (ISGs) and directs a multifaceted antiviral response. ISGs can also be activated through interferon-independent pathways, although the precise mechanisms remain elusive. Here we found that the cytosolic exonuclease Trex1 regulated the activation of a subset of ISGs independently of interferon. Both Trex1−/− mouse cells and Trex1-mutant human cells had high expression of genes encoding antiviral molecules ('antiviral genes') and were refractory to viral infection. The interferon-independent activation of antiviral genes in Trex1−/− cells required the adaptor STING, the kinase TBK1 and the transcription factors IRF3 and IRF7. We also found that Trex1-deficient cells had an expanded lysosomal compartment, altered subcellular localization of the transcription factor TFEB and diminished activity of the regulator mTORC1. Together our data identify Trex1 as a regulator of lysosomal biogenesis and interferon-independent activation of antiviral genes and show that dysregulation of lysosomes can elicit innate immune responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Iwasaki, A. & Medzhitov, R. Regulation of adaptive immunity by the innate immune system. Science 327, 291–295 (2010).
Barber, G.N. Cytoplasmic DNA innate immune pathways. Immunol. Rev. 243, 99–108 (2011).
Bowie, A.G. & Unterholzner, L. Viral evasion and subversion of pattern-recognition receptor signalling. Nat. Rev. Immunol. 8, 911–922 (2008).
Takaoka, A. et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448, 501–505 (2007).
Unterholzner, L. et al. IFI16 is an innate immune sensor for intracellular DNA. Nat. Immunol. 11, 997–1004 (2010).
Zhang, Z. et al. The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nat. Immunol. 12, 959–965 (2011).
Burdette, D.L. et al. STING is a direct innate immune sensor of cyclic di-GMP. Nature 478, 515–518 (2011).
Chen, H. et al. Activation of STAT6 by STING is critical for antiviral innate immunity. Cell 147, 436–446 (2011).
Noyce, R.S., Collins, S.E. & Mossman, K.L. Identification of a novel pathway essential for the immediate-early, interferon-independent antiviral response to enveloped virions. J. Virol. 80, 226–235 (2006).
Dixit, E. et al. Peroxisomes are signaling platforms for antiviral innate immunity. Cell 141, 668–681 (2010).
Yan, N., Regalado-Magdos, A.D., Stiggelbout, B., Lee-Kirsch, M.A. & Lieberman, J. The cytosolic exonuclease TREX1 inhibits the innate immune response to human immunodeficiency virus type 1. Nat. Immunol. 11, 1005–1013 (2010).
Gall, A. et al. Autoimmunity initiates in nonhematopoietic cells and progresses via lymphocytes in an interferon-dependent autoimmune disease. Immunity 36, 120–131 (2012).
Crow, Y. et al. Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 cause Aicardi-Goutieres syndrome at the AGS1 locus. Nat. Genet. 38, 917–920 (2006).
Crow, Y.J. & Rehwinkel, J. Aicardi-Goutieres syndrome and related phenotypes: linking nucleic acid metabolism with autoimmunity. Hum. Mol. Genet. 18, R130–R136 (2009).
Lee-Kirsch, M.A. et al. Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 are associated with systemic lupus erythematosus. Nat. Genet. 39, 1065–1067 (2007).
Lee-Kirsch, M.A. et al. A mutation in TREX1 that impairs susceptibility to granzyme A-mediated cell death underlies familial chilblain lupus. J. Mol. Med. 85, 531–537 (2007).
Richards, A. et al. C-terminal truncations in human 3′-5′ DNA exonuclease TREX1 cause autosomal dominant retinal vasculopathy with cerebral leukodystrophy. Nat. Genet. 39, 1068–1070 (2007).
Moser, K.L., Kelly, J.A., Lessard, C.J. & Harley, J.B. Recent insights into the genetic basis of systemic lupus erythematosus. Genes Immun. 10, 373–379 (2009).
Stetson, D.B., Ko, J.S., Heidmann, T. & Medzhitov, R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell 134, 587–598 (2008).
Yang, Y., Lindahl, T. & Barnes, D. Trex1 exonuclease degrades ssDNA to prevent chronic checkpoint activation and autoimmune disease. Cell 131, 873–886 (2007).
Das, S.C., Nayak, D., Zhou, Y. & Pattnaik, A.K. Visualization of intracellular transport of vesicular stomatitis virus nucleocapsids in living cells. J. Virol. 80, 6368–6377 (2006).
Orebaugh, C.D., Fye, J.M., Harvey, S., Hollis, T. & Perrino, F.W. The TREX1 exonuclease R114H mutation in Aicardi-Goutieres syndrome and lupus reveals dimeric structure requirements for DNA degradation activity. J. Biol. Chem. 286, 40246–40254 (2011).
Kreijtz, J.H.C.M., Fouchier, R.A.M. & Rimmelzwaan, G.F. Immune responses to influenza virus infection. Virus Res. 162, 19–30 (2011).
Pichlmair, A. et al. IFIT1 is an antiviral protein that recognizes 5′-triphosphate RNA. Nat. Immunol. 12, 624–630 (2011).
Yan, N. & Chen, Z.J. Intrinsic antiviral immunity. Nat. Immunol. 13, 214–222 (2012).
Brass, A.L. et al. The IFITM proteins mediate cellular resistance to influenza A H1N1 virus, West Nile virus, and dengue virus. Cell 139, 1243–1254 (2009).
Ramantani, G. et al. Expanding the phenotypic spectrum of lupus erythematosus in aicardi-goutières syndrome. Arthritis Rheum. 62, 1469–1477 (2010).
Paladino, P., Cummings, D.T., Noyce, R.S. & Mossman, K.L. The IFN-independent response to virus particle entry provides a first line of antiviral defense that is independent of TLRs and retinoic acid-inducible gene I. J. Immunol. 177, 8008–8016 (2006).
Saftig, P. & Klumperman, J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat. Rev. Mol. Cell Biol. 10, 623–635 (2009).
Cox, T.M. & Cachón-González, M.B. The cellular pathology of lysosomal diseases. J. Pathol. 226, 241–254 (2012).
Palmieri, M. et al. Characterization of the CLEAR network reveals an integrated control of cellular clearance pathways. Hum. Mol. Genet. 20, 3852–3866 (2011).
Sardiello, M. et al. A gene network regulating lysosomal biogenesis and function. Science 325, 473–477 (2009).
Settembre, C. et al. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 31, 1095–1108 (2012).
Peña-Llopis, S. et al. Regulation of TFEB and V-ATPases by mTORC1. EMBO J. 30, 3242–3258 (2011).
Roczniak-Ferguson, A. et al. The transcription factor TFEB links mTORC1 Signaling to transcriptional control of lysosome homeostasis. Sci. Signal. 5, ra42 (2012).
Vincent, M.J. et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2, 69 (2005).
Ooi, E.E., Chew, J.S., Loh, J.P. & Chua, R.C. In vitro inhibition of human influenza A virus replication by chloroquine. Virol. J. 3, 39 (2006).
Savarino, A., Boelaert, J.R., Cassone, A., Majori, G. & Cauda, R. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect. Dis. 3, 722–727 (2003).
Martina, J.A., Chen, Y., Gucek, M. & Puertollano, R. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy 8, 903–914 (2012).
Mata, M.A. et al. Chemical inhibition of RNA viruses reveals REDD1 as a host defense factor. Nat. Chem. Biol. 7, 712–719 (2011).
Grandvaux, N. et al. Transcriptional profiling of interferon regulatory factor 3 target genes: direct involvement in the regulation of interferon-stimulated genes. J. Virol. 76, 5532–5539 (2002).
Medina, D.L. et al. Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Dev. Cell 21, 421–430 (2011).
Migliorini, A. & Anders, H.-J. A novel pathogenetic concept-antiviral immunity in lupus nephritis. Nat. Rev. Nephrol. 8, 183–189 (2012).
Holm, C.K. et al. Virus-cell fusion as a trigger of innate immunity dependent on the adaptor STING. Nat. Immunol. 13, 737–743 (2012).
Marshak-Rothstein, A. Toll-like receptors in systemic autoimmune disease. Nat. Rev. Immunol. 6, 823–835 (2006).
Chiu, Y.-H., Macmillan, J.B. & Chen, Z.J. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 138, 576–591 (2009).
Keller, B.C. et al. Resistance to α/β interferon is a determinant of West Nile virus replication fitness and virulence. J. Virol. 80, 9424–9434 (2006).
Acknowledgements
We thank D. Stetson (University of Washington) for wild-type, Trex1−/− and Trex1−/−Irf3−/− primary MEFs and Trex1+/− mice, under agreement with D. Barnes and T. Lindahl (Cancer Research UK); R. Orchard and N. Alto for assistance with live cell confocal microscopy; R. Sumpter for assistance with fluorescence microscopy; Z.J. Chen (UT Southwestern Medical Center) for Sendai virus, Ifnar1−/− MEFs and discussions; M. Gale (University of Washington) for West Nile virus; B. Fontoura (UT Southwestern Medical Center) for influenza virus; Z. Zou for technical assistance; M. Whitt (University of Tennessee Health Science Center) for antibody to VSV; the Electron Microscopy Laboratory at Children's Medical Center for electron microscopy; J. Lieberman for critical reading of the manuscript; and members of the Yan laboratory for discussions. Supported by UT Southwestern Medical Center (N.Y.), the US National Institutes of Health (AI093795 and AI098569 to N.Y.; CA129387 to J.B.; and AI057156 to B.L.), The Alliance for Lupus Research (N.Y.) and Deutsche Forschungsgemeinschaft (LE 1074/4-1 to M.A.L.-K.).
Author information
Authors and Affiliations
Contributions
M.H. and N.Y. designed and did most of the experiments; J.K. helped with the experiments; M.A.L.-K. provided human cells and advice; D.R. did electron microscopy; A.K.P., J.B. and B.L. contributed reagents and advice; E.K.W. and I.D. helped analyze the data; and M.H. and N.Y. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–14 and Tables 1–2 (PDF 2001 kb)
Rights and permissions
About this article
Cite this article
Hasan, M., Koch, J., Rakheja, D. et al. Trex1 regulates lysosomal biogenesis and interferon-independent activation of antiviral genes. Nat Immunol 14, 61–71 (2013). https://doi.org/10.1038/ni.2475
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2475
This article is cited by
-
Three Prime Repair Exonuclease 1 (TREX1) expression correlates with cervical cancer cells growth in vitro and disease progression in vivo
Scientific Reports (2019)
-
Constitutive interferon signaling maintains critical threshold of MLKL expression to license necroptosis
Cell Death & Differentiation (2019)
-
A bioactive mammalian disaccharide associated with autoimmunity activates STING-TBK1-dependent immune response
Nature Communications (2019)
-
A novel autophagy enhancer as a therapeutic agent against metabolic syndrome and diabetes
Nature Communications (2018)
-
IRF3 and type I interferons fuel a fatal response to myocardial infarction
Nature Medicine (2017)