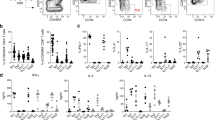
Abstract
Toll-like receptors (TLRs) sense microbial products and initiate adaptive immune responses by activating dendritic cells (DCs). As pathogens may contain several TLR agonists, we sought to determine whether different TLRs cooperate in DC activation. In human and mouse DCs, TLR3 and TLR4 potently acted in synergy with TLR7, TLR8 and TLR9 in the induction of a selected set of genes. Synergic TLR stimulation increased production of interleukins 12 and 23 and increased the Delta-4/Jagged-1 ratio, leading to DCs with enhanced and sustained T helper type 1–polarizing capacity. Global gene transcriptional analysis showed that TLR synergy 'boosted' only approximately 1% of the transcripts induced by single TLR agonists. These results identify a 'combinatorial code' by which DCs discriminate pathogens and suggest new strategies for promoting T helper type 1 responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
References
Iwasaki, A. & Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5, 987–995 (2004).
Takeda, K., Kaisho, T. & Akira, S. Toll-like receptors. Annu. Rev. Immunol. 21, 335–376 (2003).
Medzhitov, R., Preston-Hurlburt, P. & Janeway, C.A., Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388, 394–397 (1997).
Poltorak, A. et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088 (1998).
Hemmi, H. et al. A Toll-like receptor recognizes bacterial DNA. Nature 408, 740–745 (2000).
Alexopoulou, L., Holt, A.C., Medzhitov, R. & Flavell, R.A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413, 732–738 (2001).
Akira, S. & Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 4, 499–511 (2004).
Yamamoto, M. et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301, 640–643 (2003).
Oshiumi, H., Matsumoto, M., Funami, K., Akazawa, T. & Seya, T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat. Immunol. 4, 161–167 (2003).
Horng, T., Barton, G.M. & Medzhitov, R. TIRAP: an adapter molecule in the Toll signaling pathway. Nat. Immunol. 2, 835–841 (2001).
Meylan, E. et al. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-κB activation. Nat. Immunol. 5, 503–507 (2004).
Yamamoto, M. et al. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat. Immunol. 4, 1144–1150 (2003).
Bin, L.H., Xu, L.G. & Shu, H.B. TIRP, a novel Toll/interleukin-1 receptor (TIR) domain-containing adapter protein involved in TIR signaling. J. Biol. Chem. 278, 24526–24532 (2003).
Oshiumi, H. et al. TIR-containing adapter molecule (TICAM)-2, a bridging adapter recruiting to Toll-like receptor 4 TICAM-1 that induces interferon-β. J. Biol. Chem. 278, 49751–49762 (2003).
Ahmad-Nejad, P. et al. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur. J. Immunol. 32, 1958–1968 (2002).
Heil, F. et al. The Toll-like receptor 7 (TLR7)-specific stimulus loxoribine uncovers a strong relationship within the TLR7, 8 and 9 subfamily. Eur. J. Immunol. 33, 2987–2997 (2003).
Kadowaki, N. et al. Subsets of human dendritic cell precursors express different Toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194, 863–869 (2001).
Jarrossay, D., Napolitani, G., Colonna, M., Sallusto, F. & Lanzavecchia, A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur. J. Immunol. 31, 3388–3393 (2001).
Bernasconi, N.L., Onai, N. & Lanzavecchia, A. A role for Toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood 101, 4500–4504 (2003).
Banchereau, J. & Steinman, R.M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3, 133–146 (2003).
Schulz, O. et al. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity 13, 453–462 (2000).
Snijders, A., Kalinski, P., Hilkens, C.M. & Kapsenberg, M.L. High-level IL-12 production by human dendritic cells requires two signals. Int. Immunol. 10, 1593–1598 (1998).
Edwards, A.D. et al. Microbial recognition via Toll-like receptor-dependent and -independent pathways determines the cytokine response of murine dendritic cell subsets to CD40 triggering. J. Immunol. 169, 3652–3660 (2002).
Reis e Sousa, C. Activation of dendritic cells: translating innate into adaptive immunity. Curr. Opin. Immunol. 16, 21–25 (2004).
Oppmann, B. et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13, 715–725 (2000).
Cua, D.J. et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421, 744–748 (2003).
Langrish, C.L. et al. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol. Rev. 202, 96–105 (2004).
Amsen, D. et al. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell 117, 515–526 (2004).
Gao, J.J. et al. Cutting edge: bacterial DNA and LPS act in synergy in inducing nitric oxide production in RAW 264.7 macrophages. J. Immunol. 163, 4095–4099 (1999).
Sato, S. et al. Synergy and cross-tolerance between toll-like receptor (TLR) 2- and TLR4-mediated signaling pathways. J. Immunol. 165, 7096–7101 (2000).
Yi, A.K., Yoon, J.G., Hong, S.C., Redford, T.W. & Krieg, A.M. Lipopolysaccharide and CpG DNA synergize for tumor necrosis factor-α production through activation of NF-κB. Int. Immunol. 13, 1391–1404 (2001).
Jurk, M. et al. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat. Immunol. 3, 499 (2002).
Lee, J. et al. Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: activation of Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 100, 6646–6651 (2003).
Langenkamp, A., Messi, M., Lanzavecchia, A. & Sallusto, F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat. Immunol. 1, 311–316 (2000).
Sato, S. et al. A variety of microbial components induce tolerance to lipopolysaccharide by differentially affecting MyD88-dependent and -independent pathways. Int. Immunol. 14, 783–791 (2002).
Yoshimori, T., Yamamoto, A., Moriyama, Y., Futai, M. & Tashiro, Y. Bafilomycin A1, a specific inhibitor of vacuolar-type H+-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J. Biol. Chem. 266, 17707–17712 (1991).
Forster, R. et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99, 23–33 (1999).
Martinon, F., Agostini, L., Meylan, E. & Tschopp, J. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr. Biol. 14, 1929–1934 (2004).
Martinon, F. & Tschopp, J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell 117, 561–574 (2004).
Yamamoto, M. et al. Regulation of Toll/IL-1-receptor-mediated gene expression by the inducible nuclear protein IκB-ζ. Nature 430, 218–222 (2004).
Yao, J., Mackman, N., Edgington, T.S. & Fan, S.T. Lipopolysaccharide induction of the tumor necrosis factor-α promoter in human monocytic cells. Regulation by Egr-1, c-Jun, and NF-κB transcription factors. J. Biol. Chem. 272, 17795–17801 (1997).
Napolitani, G., Bortoletto, N., Racioppi, L., Lanzavecchia, A. & D'Oro, U. Activation of src-family tyrosine kinases by LPS regulates cytokine production in dendritic cells by controlling AP-1 formation. Eur. J. Immunol. 33, 2832–2841 (2003).
Nakahara, T. et al. Role of c-Jun N-terminal kinase on lipopolysaccharide induced maturation of human monocyte-derived dendritic cells. Int. Immunol. 16, 1701–1709 (2004).
Sallusto, F. & Lanzavecchia, A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J. Exp. Med. 179, 1109–1118 (1994).
Lutz, M.B. et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223, 77–92 (1999).
Acknowledgements
We thank G. Natoli and A. Macagno for critical reading and A. Martín-Fontecha for help with the experiments using mouse DCs. Supported in part by the Swiss National Science Foundation (31-63885), National Institutes of Health (U19AI057266/01) and European Community (Sixth Framework Programme, contract LSHP-CT-2003-503240, Mucosal Vaccines for Poverty-Related Diseases (MUVAPRED)).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Syngergistic TLR stimulation does not affect the extent of MHC and B7 upregulation. (PDF 56 kb)
Supplementary Fig. 2
Exogenous IFN-β has only a modest effect on IL-12p70 production irrespective of the nature of the maturation stimulus. (PDF 26 kb)
Supplementary Fig. 3
The synergistic effect of R848 on LPS induced IL-12 production requires endosomal acidification. (PDF 57 kb)
Supplementary Fig. 4
TLR synergy increases production of TNF, IL-6 and IL-10. (PDF 25 kb)
Rights and permissions
About this article
Cite this article
Napolitani, G., Rinaldi, A., Bertoni, F. et al. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1–polarizing program in dendritic cells. Nat Immunol 6, 769–776 (2005). https://doi.org/10.1038/ni1223
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1223
This article is cited by
-
Emerging role of microbiota derived outer membrane vesicles to preventive, therapeutic and diagnostic proposes
Infectious Agents and Cancer (2023)
-
A nanoadjuvant that dynamically coordinates innate immune stimuli activation enhances cancer immunotherapy and reduces immune cell exhaustion
Nature Nanotechnology (2023)
-
RETRACTED ARTICLE: Selected TLR7/8 agonist and type I interferon (IFN-α) cooperatively redefine the microglia transcriptome
Inflammopharmacology (2023)
-
Bacterial couriers as cancer vaccines
Nature Biomedical Engineering (2022)
-
Blood DCs activated with R848 and poly(I:C) induce antigen-specific immune responses against viral and tumor-associated antigens
Cancer Immunology, Immunotherapy (2022)