Abstract
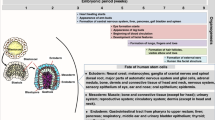
The evolutionary adaptation in mammals that allows implantation of their embryos in the mother's womb creates an immunological problem. Although it ensures optimal nourishment and protection of the fetus throughout its early development, intimate contact with the mother's uterine tissue makes the fetus a potential target for her immune system. As half the fetal genes are derived from the father, the developing embryo and placenta must be considered a 'semi-allograft'. Such a mismatched organ transplant would be readily rejected without powerful immune suppression. During pregnancy, however, the semi-allogeneic fetus is protected from assault by the maternal immune system over an extended period of time. The mother's immune system seems to recognize the fetus as 'temporary self'. How this feat is managed is key to understanding immunological tolerance and intervention in treating disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Heard, D.H., Hinde, I.T. & Mynors, L.S. An experimental study of haemolytic disease of the newborn due to isoimmunization of pregnancy. J. Hyg. (Lond.) 47, 119–131 (1949).
Medawar, P.B. Some immunological and endocrinological problems raised by evolution of viviparity in vertebrates. Symp. Soc. Exp. Biol. 7, 320–328 (1953).
Billingham, R.E., Brent, L. & Medawar, P.B. Activity acquired tolerance of foreign cells. Nature 172, 603–606 (1953).
Owen, R.D. Immunogenetic consequences of vascular anastomoses between bovine twins. Science 102, 400–401 (1945).
Medawar, P.B. & Hunt, R. Vulnerability of methylcholanthrene-induced tumours to immunity aroused by syngeneic foetal cells. Nature 271, 164–165 (1978).
Hammarstrom, S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 9, 67–81 (1999).
Abrahams, V.M. & Mor, G. Toll-like receptors and their role in the trophoblast. Placenta 26, 540–547 (2005).
Stallmach, T. & Hebisch, G. Placental pathology: its impact on explaining prenatal and perinatal death. Virchows Arch. 445, 9–16 (2004).
Haque, A. & Capron, A. Transplacental transfer of rodent microfilariae induces antigen-specific tolerance in rats. Nature 299, 361–363 (1982).
Pearson, H. Reproductive immunology: Immunity's pregnant pause. Nature 420, 265–266 (2002).
Bertrams, J., Kuwert, E. & Lohmeyer, H.H. The specificity of leukocyte antibodies in histocompatibility testing of serum from prima- and multiparas. Bibl. Haematol. 37, 98–106 (1971).
Brambell, F.W.R., Hemmings, W.A. & Henderson, M. in Antibodies and Embryos (Athlone Press, University of London, 1951).
Medawar, P.B. & Sparrow, E.M. The effects of adrenocortical hormones, adrenocorticotrophic hormone and pregnancy on skin transplantation immunity in mice. J. Endocrinol. 14, 240–256 (1956).
Chaouat, G. & Voisin, G.A. Regulatory T cell subpopulations in pregnancy. I. Evidence for suppressive activity of the early phase of MLR. J. Immunol. 122, 1383–1388 (1979).
Aluvihare, V.R., Kallikourdis, M. & Betz, A.G. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 5, 266–271 (2004).
Tafuri, A., Alferink, J., Moller, P., Hammerling, G.J. & Arnold, B. T cell awareness of paternal alloantigens during pregnancy. Science 270, 630–633 (1995).
Moffett-King, A. Natural killer cells and pregnancy. Nat. Rev. Immunol. 2, 656–663 (2002).
Rossant, J. & Cross, J.C. Placental development: lessons from mouse mutants. Nat. Rev. Genet. 2, 538–548 (2001).
Streilein, J.W. Peripheral tolerance induction: lessons from immune privileged sites and tissues. Transplant. Proc. 28, 2066–2070 (1996).
Ferguson, T.A. & Griffith, T.S. A vision of cell death: insights into immune privilege. Immunol. Rev. 156, 167–184 (1997).
Aoki, K. et al. Extracellular matrix interacts with soluble CD95L: retention and enhancement of cytotoxicity. Nat. Immunol. 2, 333–337 (2001).
Kamimura, S., Eguchi, K., Yonezawa, M. & Sekiba, K. Localization and developmental change of indoleamine 2,3-dioxygenase activity in the human placenta. Acta Med. Okayama 45, 135–139 (1991).
Munn, D.H. et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 281, 1191–1193 (1998).
Baban, B. et al. Indoleamine 2,3-dioxygenase expression is restricted to fetal trophoblast giant cells during murine gestation and is maternal genome specific. J. Reprod. Immunol. 61, 67–77 (2004).
Hunt, J.S., Vassmer, D., Ferguson, T.A. & Miller, L. Fas ligand is positioned in mouse uterus and placenta to prevent trafficking of activated leukocytes between the mother and the conceptus. J. Immunol. 158, 4122–4128 (1997).
Xu, C. et al. A critical role for murine complement regulator crry in fetomaternal tolerance. Science 287, 498–501 (2000).
Rooney, I.A., Oglesby, T.J. & Atkinson, J.P. Complement in human reproduction: activation and control. Immunol. Res. 12, 276–294 (1993).
Piccinni, M.P. et al. Defective production of both leukemia inhibitory factor and type 2 T-helper cytokines by decidual T cells in unexplained recurrent abortions. Nat. Med. 4, 1020–1024 (1998).
Lin, H., Mosmann, T.R., Guilbert, L., Tuntipopipat, S. & Wegmann, T.G. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J. Immunol. 151, 4562–4573 (1993).
Wegmann, T.G., Lin, H., Guilbert, L. & Mosmann, T.R. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol. Today 14, 353–356 (1993).
Fallon, P.G. et al. IL-4 induces characteristic Th2 responses even in the combined absence of IL-5, IL-9, and IL-13. Immunity 17, 7–17 (2002).
Mattsson, R. The non-expression of MHC class II in trophoblast cells. Am. J. Reprod. Immunol. 40, 383–384 (1998).
van den Elsen, P.J., Holling, T.M., Kuipers, H.F. & van der Stoep, N. Transcriptional regulation of antigen presentation. Curr. Opin. Immunol. 16, 67–75 (2004).
Peyman, J.A., Nelson, P.J. & Hammond, G.L. HLA-DR genes are silenced in human trophoblasts and stimulation of signal transduction pathways does not circumvent interferon-γ unresponsiveness. Transplant. Proc. 24, 470–471 (1992).
Goodfellow, P.N., Barnstable, C.J., Bodmer, W.F., Snary, D. & Crumpton, M.J. Expression of HLA system antigens on placenta. Transplantation 22, 595–603 (1976).
Fruh, K., Gruhler, A., Krishna, R.M. & Schoenhals, G.J. A comparison of viral immune escape strategies targeting the MHC class I assembly pathway. Immunol. Rev. 168, 157–166 (1999).
Kovats, S. et al. A class I antigen, HLA-G, expressed in human trophoblasts. Science 248, 220–223 (1990).
Ishitani, A. et al. Re-examination of HLA-G polymorphism in African Americans. Immunogenetics 49, 808–811 (1999).
Collins, K.L. & Baltimore, D. HIV's evasion of the cellular immune response. Immunol. Rev. 168, 65–74 (1999).
Hiby, S.E. et al. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J. Exp. Med. 200, 957–965 (2004).
Parham, P. NK cells and trophoblasts: partners in pregnancy. J. Exp. Med. 200, 951–955 (2004).
Navarro, F. et al. The ILT2 (LIR1) and CD94/NKG2A NK cell receptors respectively recognize HLA-G1 and HLA-E molecules co-expressed on target cells. Eur. J. Immunol. 29, 277–283 (1999).
Rajagopalan, S. & Long, E.O. A human histocompatibility leukocyte antigen (HLA)-G-specific receptor expressed on all natural killer cells. J. Exp. Med. 189, 1093–1100 (1999).
Rajagopalan, S. et al. Activation of NK cells by an endocytosed receptor for soluble HLA-G. PLoS Biol. (in the press) (2006).
Colonna, M., Nakajima, H., Navarro, F. & Lopez-Botet, M. A novel family of Ig-like receptors for HLA class I molecules that modulate function of lymphoid and myeloid cells. J. Leukoc. Biol. 66, 375–381 (1999).
Shiroishi, M. et al. Human inhibitory receptors Ig-like transcript 2 (ILT2) and ILT4 compete with CD8 for MHC class I binding and bind preferentially to HLA-G. Proc. Natl. Acad. Sci. USA 100, 8856–8861 (2003).
Chang, C.C. et al. Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat. Immunol. 3, 237–243 (2002).
Ashkar, A.A. et al. Assessment of requirements for IL-15 and IFN regulatory factors in uterine NK cell differentiation and function during pregnancy. J. Immunol. 171, 2937–2944 (2003).
Nelson, J.L. Microchimerism in human health and disease. Autoimmunity 36, 5–9 (2003).
Huppertz, B. & Kingdom, J.C. Apoptosis in the trophoblast–role of apoptosis in placental morphogenesis. J. Soc. Gynecol. Investig. 11, 353–362 (2004).
Arck, P.C., Ferrick, D.A., Steele-Norwood, D., Croitoru, K. & Clark, D.A. Murine T cell determination of pregnancy outcome: I. Effects of strain, αβ T cell receptor, γδ T cell receptor, and γδ T cell subsets. Am. J. Reprod. Immunol. 37, 492–502 (1997).
Jin, L.P. et al. Adoptive transfer of paternal antigen-hyporesponsive T cells induces maternal tolerance to the allogeneic fetus in abortion-prone matings. J. Immunol. 173, 3612–3619 (2004).
Blois, S. et al. Therapy with dendritic cells influences the spontaneous resorption rate in the CBA/J x DBA/2J mouse model. Am. J. Reprod. Immunol. 51, 40–48 (2004).
Zenclussen, A.C. CD4+CD25+ T regulatory cells in murine pregnancy. J. Reprod. Immunol. 65, 101–110 (2005).
Chaouat, G., Voisin, G.A., Daeron, M. & Kanellopoulos, J. Enhancing antibodies and supressive cells in maternal anti-fetal immune reaction. Ann. Immunol. (Paris) 128, 21–24 (1977).
Moller, G. Do suppressor T cells exist? Scand. J. Immunol. 27, 247–250 (1988).
Eichmann, K. Suppression needs a new hypothesis. Scand. J. Immunol. 28, 273–276 (1988).
Sakaguchi, S., Fukuma, K., Kuribayashi, K. & Masuda, T. Organ-specific autoimmune diseases induced in mice by elimination of T cell subset. I. Evidence for the active participation of T cells in natural self-tolerance; deficit of a T cell subset as a possible cause of autoimmune disease. J. Exp. Med. 161, 72–87 (1985).
Sakaguchi, S., Sakaguchi, N., Asano, M., Itoh, M. & Toda, M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155, 1151–1164 (1995).
Fontenot, J.D., Gavin, M.A. & Rudensky, A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4, 330–336 (2003).
Hori, S., Nomura, T. & Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061 (2003).
Khattri, R., Cox, T., Yasayko, S.A. & Ramsdell, F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4, 337–342 (2003).
Godfrey, V.L., Wilkinson, J.E., Rinchik, E.M. & Russell, L.B. Fatal lymphoreticular disease in the scurfy (sf) mouse requires T cells that mature in a sf thymic environment: potential model for thymic education. Proc. Natl. Acad. Sci. USA 88, 5528–5532 (1991).
Bennett, C.L. et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 27, 20–21 (2001).
Wildin, R.S. et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 27, 18–20 (2001).
Mattsson, R., Mattsson, A., Holmdahl, R., Whyte, A. & Rook, G.A. Maintained pregnancy levels of oestrogen afford complete protection from post-partum exacerbation of collagen-induced arthritis. Clin. Exp. Immunol. 85, 41–47 (1991).
Ostensen, M. & Villiger, P.M. Immunology of pregnancy-pregnancy as a remission inducing agent in rheumatoid arthritis. Transpl. Immunol. 9, 155–160 (2002).
Beagley, K.W. & Gockel, C.M. Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol. Med. Microbiol. 38, 13–22 (2003).
Condrea, P., Poenaru, E., Esrig, M. & Filipesco, I. Antitetanus immunization of the newborn infant by vaccination during pregnancy. Clinical and experimental studies. Arch. Roum. Pathol. Exp. Microbiol. 20, 549–564 (1961).
Heikkinen, J., Mottonen, M., Alanen, A. & Lassila, O. Phenotypic characterization of regulatory T cells in the human decidua. Clin. Exp. Immunol. 136, 373–378 (2004).
Sasaki, Y. et al. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol. Hum. Reprod. 10, 347–353 (2004).
Somerset, D.A., Zheng, Y., Kilby, M.D., Sansom, D.M. & Drayson, M.T. Normal human pregnancy is associated with an elevation in the immune suppressive CD25 CD4 regulatory T-cell subset. Immunology 112, 38–43 (2004).
Matzinger, P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 12, 991–1045 (1994).
Shao, L., Jacobs, A.R., Johnson, V.V. & Mayer, L. Activation of CD8+ regulatory T cells by human placental trophoblasts. J. Immunol. 174, 7539–7547 (2005).
Acknowledgements
We thank G. Gruetz, M. Kallikourdis and A. Moffett for critical reading of the manuscript, and E. Long and S. Rajagopalan for access to data in the press. Supported by the Wellcome Trust (J.T.) and the Medical Research Council (J.T. and A.G.B.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Trowsdale, J., Betz, A. Mother's little helpers: mechanisms of maternal-fetal tolerance. Nat Immunol 7, 241–246 (2006). https://doi.org/10.1038/ni1317
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1317
This article is cited by
-
Evaluation of changes in exhausted T lymphocytes and miRNAs expression in the different trimesters of pregnancy in pregnant women
Molecular Biology Reports (2024)
-
Prevention of intrauterine fetal growth restriction by administrating C1q/TNF-related protein 6, a specific inhibitor of the alternative complement pathway
Journal of Assisted Reproduction and Genetics (2022)
-
Attenuated asthma phenotype in mice with a fetal-like antigen receptor repertoire
Scientific Reports (2021)
-
Immunological considerations and challenges for regenerative cellular therapies
Communications Biology (2021)
-
Immunological and physiopathological approach of COVID-19 in pregnancy
Archives of Gynecology and Obstetrics (2021)