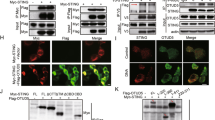
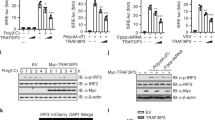
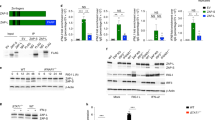
Abstract
Recognition of double-stranded RNA activates interferon-regulatory factor 3 (IRF3)–dependent expression of antiviral factors. Although the molecular mechanisms underlying the activation of IRF3 have been studied, the mechanisms by which IRF3 activity is reduced have not. Here we report that activation of IRF3 is negatively regulated by the peptidyl-prolyl isomerase Pin1. After stimulation by double-stranded RNA, induced phosphorylation of the Ser339–Pro340 motif of IRF3 led to its interaction with Pin1 and finally polyubiquitination and then proteasome-dependent degradation of IRF3. Suppression of Pin1 by RNA interference or genetic deletion resulted in enhanced IRF-3-dependent production of interferon-β, with consequent reduction of virus replication. These results elucidate a previously unknown mechanism for controlling innate antiviral responses by negatively regulating IRF3 activity via Pin1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Taniguchi, T., Ogasawara, K., Takaoka, A. & Tanaka, N. IRF family of transcription factors as regulators of host defense. Annu. Rev. Immunol. 19, 623–655 (2001).
Hiscott, J. et al. Convergence of the NF-κB and interferon signaling pathways in the regulation of antiviral defense and apoptosis. Ann. NY Acad. Sci. 1010, 237–248 (2003).
Takeda, K. & Akira, S. Toll-like receptors in innate immunity. Int. Immunol. 17, 1–14 (2005).
Alexopoulou, L., Holt, A.C., Medzhitov, R. & Flavell, R.A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413, 732–738 (2001).
Yoneyama, M. et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5, 730–737 (2004).
Poltorak, A. et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088 (1998).
Doyle, S. et al. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity 17, 251–263 (2002).
Yoneyama, M. et al. Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF3 and CBP/p300. EMBO J. 17, 1087–1095 (1998).
Mori, M. et al. Identification of Ser-386 of interferon regulatory factor 3 as critical target for inducible phosphorylation that determines activation. J. Biol. Chem. 279, 9698–9702 (2004).
Lin, R., Heylbroeck, C., Pitha, P.M. & Hiscott, J. Virus-dependent phosphorylation of the IRF3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Mol. Cell. Biol. 18, 2986–2996 (1998).
Lin, R., Mamane, Y. & Hiscott, J. Structural and functional analysis of interferon regulatory factor 3: localization of the transactivation and autoinhibitory domains. Mol. Cell. Biol. 19, 2465–2474 (1999).
Servant, M.J. et al. Identification of distinct signaling pathways leading to the phosphorylation of interferon regulatory factor 3. J. Biol. Chem. 276, 355–363 (2001).
Karpova, A.Y., Trost, M., Murray, J.M., Cantley, L.C. & Howley, P.M. Interferon regulatory factor-3 is an in vivo target of DNA-PK. Proc. Natl. Acad. Sci. USA 99, 2818–2823 (2002).
Fitzgerald, K.A. et al. IKKε and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4, 491–496 (2003).
Sharma, S. et al. Triggering the interferon antiviral response through an IKK-related pathway. Science 300, 1148–1151 (2003).
McWhirter, S.M. et al. IFN-regulatory factor 3-dependent gene expression is defective in Tbk1-deficient mouse embryonic fibroblasts. Proc. Natl. Acad. Sci. USA 101, 233–238 (2004).
Hemmi, H. et al. The roles of two IκB kinase-related kinases in lipopolysaccharide and double stranded RNA signaling and viral infection. J. Exp. Med. 199, 1641–1650 (2004).
Wulf, G., Finn, G., Suizu, F. & Lu, K.P. Phosphorylation-specific prolyl isomerization: is there an underlying theme? Nat. Cell Biol. 7, 435–441 (2005).
Lu, K.P., Hanes, S.D. & Hunter, T. A human peptidyl-prolyl isomerase essential for regulation of mitosis. Nature 380, 544–547 (1996).
Ryo, A. et al. Pin1 regulates turnover and subcellular localization of β-catenin by inhibiting its interaction with APC. Nat. Cell Biol. 3, 793–801 (2001).
Wulf, G.M., Liou, Y.C., Ryo, A., Lee, S.W. & Lu, K.P. Role of Pin1 in the regulation of p53 stability and p21 transactivation, and cell cycle checkpoints in response to DNA damage. J. Biol. Chem. 277, 47976–47979 (2002).
Zacchi, P. et al. The prolyl isomerase Pin1 reveals a mechanism to control p53 functions after genotoxic insults. Nature 419, 853–857 (2002).
Zheng, H. et al. The prolyl isomerase Pin1 is a regulator of p53 in genotoxic response. Nature 419, 849–853 (2002).
Ryo, A. et al. PIN1 is an E2F target gene essential for Neu/Ras-induced transformation of mammary epithelial cells. Mol. Cell. Biol. 22, 5281–5295 (2002).
Liou, Y.C. et al. Role of the prolyl isomerase Pin1 in protecting against age-dependent neurodegeneration. Nature 424, 556–561 (2003).
Ryo, A. et al. Regulation of NF-κB signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65/RelA. Mol. Cell 12, 1413–1426 (2003).
Mantovani, F. et al. Pin1 links the activities of c-Abl and p300 in regulating p73 function. Mol. Cell 14, 625–636 (2004).
Yeh, E. et al. A signalling pathway controlling c-Myc degradation that impacts oncogenic transformation of human cells. Nat. Cell Biol. 6, 308–318 (2004).
Yamamoto, M. et al. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-β promoter in the Toll-like receptor signaling. J. Immunol. 169, 6668–6672 (2002).
Saitoh, T. et al. A20 is a negative regulator of IFN regulatory factor 3 signaling. J. Immunol. 174, 1507–1512 (2005).
Sato, M. et al. Distinct and essential roles of transcription factors IRF3 and IRF-7 in response to viruses for IFN-α/β gene induction. Immunity 13, 539–548 (2000).
Lipford, J.R. & Deshaies, R.J. Diverse roles for ubiquitin-dependent proteolysis in transcriptional activation. Nat. Cell Biol. 5, 845–850 (2003).
Bao, L. et al. Prevalent overexpression of prolyl isomerase Pin1 in human cancers. Am. J. Pathol. 164, 1727–1737 (2004).
Stojdl, D.F. et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 4, 263–275 (2003).
Sakaguchi, S. et al. Essential role of IRF3 in lipopolysaccharide-induced interferon-β gene expression and endotoxin shock. Biochem. Biophys. Res. Commun. 306, 860–866 (2003).
Hsu, L.C. et al. The protein kinase PKR is required for macrophage apoptosis after activation of Toll-like receptor 4. Nature 428, 341–345 (2004).
Heylbroeck, C. et al. The IRF3 transcription factor mediates Sendai virus-induced apoptosis. J. Virol. 74, 3781–3792 (2000).
Honda, K. et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434, 772–777 (2005).
Sears, R. et al. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev. 14, 2501–2514 (2000).
Saitoh, T. et al. TWEAK induces NF-κB2 p100 processing and long lasting NF-κB activation. J. Biol. Chem. 278, 36005–36012 (2003).
Momoi, Y. et al. Pertussis toxin enhances human immunodeficiency virus type 1 replication. AIDS Res. Hum. Retroviruses 16, 373–379 (2000).
Morita, S., Kojima, T. & Kitamura, T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 7, 1063–1066 (2000).
Fujimori, F., Takahashi, K., Uchida, C. & Uchida, T. Mice lacking Pin1 develop normally, but are defective in entering cell cycle from G0 arrest. Biochem. Biophys. Res. Commun. 265, 658–663 (1999).
Acknowledgements
We thank K. Swanson for technical advice. Supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan (13226027 and 14406009 to N.Y.; 16659125 and 17013029 to S.Y.), the National Institutes of Health (GM58556 to K.P.L.), the Japan Society for the Promotion of Science (T.S.), the Swiss Foundation for Grants in Biology and Medicine (A.T.-K.) and the Leukemia and Lymphoma Society (A.R.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Rapid and transient phosphorylation of IRF3 at Ser396 versus delayed phosphorylation of IRF3 at Ser339 in response to TLR3 or TLR4 stimulation. (PDF 142 kb)
Supplementary Fig. 2
TBK1 and IKK-i are involved in phosphorylation of IRF3 at Ser339. (PDF 113 kb)
Supplementary Fig. 3
Pin1 does not significantly affect NF-κB activation induced by dsRNA stimulation or virus infection. (PDF 156 kb)
Supplementary Fig. 4
Specific inhibition of Pin1 expression augments IRF3-dependent transcriptional activation. (PDF 175 kb)
Supplementary Fig. 5
Forced expression of Pin1 or dominant negative IRF3 antagonizes the polyIC-induced antiviral activity. (PDF 1020 kb)
Supplementary Fig. 6
Pin1 expression levels following stimulation or virus infection. (PDF 133 kb)
Rights and permissions
About this article
Cite this article
Saitoh, T., Tun-Kyi, A., Ryo, A. et al. Negative regulation of interferon-regulatory factor 3–dependent innate antiviral response by the prolyl isomerase Pin1. Nat Immunol 7, 598–605 (2006). https://doi.org/10.1038/ni1347
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1347
This article is cited by
-
Lysine methyltransferase SMYD2 inhibits antiviral innate immunity by promoting IRF3 dephosphorylation
Cell Death & Disease (2023)
-
Regulation of antiviral innate immune signaling and viral evasion following viral genome sensing
Experimental & Molecular Medicine (2021)
-
Ubiquitination modification: critical regulation of IRF family stability and activity
Science China Life Sciences (2021)
-
The methyltransferase PRMT6 attenuates antiviral innate immunity by blocking TBK1–IRF3 signaling
Cellular & Molecular Immunology (2019)
-
Prolyl isomerase Pin1: a promoter of cancer and a target for therapy
Cell Death & Disease (2018)