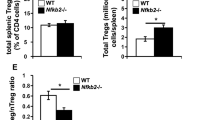
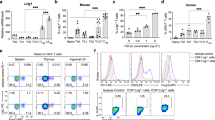
Abstract
T cell activation involves the orchestration of several signaling pathways, including that of the 'classical' transcription factor NF-κB components NF-κB1–RelA. The function of the 'nonclassical' NF-κB2–RelB pathway is less clear, although T cells lacking components of this pathway have activation defects. Here we show that mice deficient in NF-κB-inducing kinase have a complex phenotype consisting of immunosuppression mediated by CD25−Foxp3− memory CD4+ cells and, in the absence of those cells, hyper-responsive naive CD4+ T cells, which caused autoimmune lesions after adoptive transfer into hosts deficient in recombination-activating genes. Biochemical studies indicated involvement of a cell-intrinsic mechanism in which NF-κB2 (p100) limits nuclear translocation of NF-κB1–RelA and thereby functions as a regulatory 'brake' for the activation of naive T cells.
NOTE: In the version of this article initially published, the sentence on page 763, column 2, line 12 is incorrect. The correct sentence should end “…and these mice show increased susceptibility to typhlocolitis and infection with Leishmania major but are resistant to experimental autoimmune encephalomyelitis and asthma14–17.” The error has been corrected in the PDF version of the article.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
19 October 2007
In the version of this article initially published, the legends for Figures 1 and 5 are incorrect. The correct phrasing should be, for Figure 1a, “... injection of α-DEC-MCC, α-DEC-APL constructs, α-DEC-ovalbumin (α-DEC-OVA), α-DEC-205 plus isotype-MCC (α-DEC + iso-MCC) or PBS...”; for Figure 1b,c, “...injection of α-DEC-MCC, α-DEC-APL constructs, α-DEC-ovalbumin, α-DEC-205 plus isotype-MCC or PBS...”; and for Figure 5, “...mice were injected with α-DEC-APL constructs and then, 5 h later, with Fluo-4 AM– and CMRA-colabeled AND T cells” on line 2, and ‘Frequency of AND T cells with ‘sustained’ Ca2+ increase in mice treated...” on line 3. The errors have been corrected in the HTML and PDF versions of the article.
References
Ghosh, S., May, M.J. & Kopp, E.B. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16, 225–260 (1998).
Karin, M. How NF-κB is activated: the role of the IκB kinase (IKK) complex. Oncogene 18, 6867–6874 (1999).
Bonizzi, G. & Karin, M. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 25, 280–288 (2004).
Kahn-Perles, B., Lipcey, C., Lecine, P., Olive, D. & Imbert, J. Temporal and subunit-specific modulations of the Rel/NF-κB transcription factors through CD28 costimulation. J. Biol. Chem. 272, 21774–21783 (1997).
Sun, Z. et al. PKC-theta is required for TCR-induced NF-κB activation in mature but not immature T lymphocytes. Nature 404, 402–407 (2000).
Ruland, J. et al. Bcl10 is a positive regulator of antigen receptor-induced activation of NF-κB and neural tube closure. Cell 104, 33–42 (2001).
Thome, M. & Tschopp, J. TCR-induced NF-κB activation: a crucial role for Carma1, Bcl10 and MALT1. Trends Immunol. 24, 419–424 (2003).
Karin, M. & Ben-Neriah, Y. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18, 621–663 (2000).
Pimentel-Muinos, F. et al. Regulation of interleukin-2α chain expression and nuclear factor κB activation by protein kinase C in T lymphocytes. Autocrine role of tumor necrosis factor α. J. Biol. Chem. 269, 24424–24429 (1994).
Prasad, A.S. et al. Zinc enhances the expression of interleukin-2 and interleukin-2 receptors in HUT-78 cells by way of NF-κB activation. J. Lab. Clin. Med. 140, 272–289 (2002).
Dejardin, E. et al. The lymphotoxin-β receptor induces different patterns of gene expression via two NF-κB pathways. Immunity 17, 525–535 (2002).
Matsushima, A. et al. Essential role of nuclear factor (NF)-κB-inducing kinase and inhibitor of κB (IκB) kinase α in NF-κB activation through lymphotoxin β receptor, but not through tumor necrosis factor receptor I. J. Exp. Med. 193, 631–636 (2001).
Matsumoto, M. et al. Essential role of NF-κB-inducing kinase in T cell activation through the TCR/CD3 pathway. J. Immunol. 169, 1151–1158 (2002).
Artis, D. et al. NF-κB1 is required for optimal CD4+ Th1 cell development and resistance to Leishmania major. J. Immunol. 170, 1995–2003 (2003).
Erdman, S., Fox, J.G., Dangler, C.A., Feldman, D. & Horwitz, B.H. Typhlocolitis in NF-κB-deficient mice. J. Immunol. 166, 1443–1447 (2001).
Hilliard, B., Samoilova, E.B., Liu, T.S., Rostami, A. & Chen, Y. Experimental autoimmune encephalomyelitis in NF-κB-deficient mice:roles of NF-κB in the activation and differentiation of autoreactive T cells. J. Immunol. 163, 2937–2943 (1999).
Das, J. et al. A critical role for NF-κB in GATA3 expression and TH2 differentiation in allergic airway inflammation. Nat. Immunol. 2, 45–50 (2001).
Beg, A.A., Sha, W.C., Bronson, R.T., Ghosh, S. & Baltimore, D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature 376, 167–170 (1995).
Burkly, L. et al. Expression of RelB is required for the development of thymic medulla and dendritic cells. Nature 373, 531–536 (1995).
Weih, F. et al. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-κB/Rel family. Cell 80, 331–340 (1995).
Franzoso, G. et al. Mice deficient in nuclear factor (NF)-κB/p52 present with defects in humoral responses, germinal center reactions, and splenic microarchitecture. J. Exp. Med. 187, 147–159 (1998).
Speirs, K., Lieberman, L., Caamano, J., Hunter, C.A. & Scott, P. Cutting edge: NF-κB2 is a negative regulator of dendritic cell function. J. Immunol. 172, 752–756 (2004).
Hilliard, B.A. et al. Critical roles of c-Rel in autoimmune inflammation and helper T cell differentiation. J. Clin. Invest. 110, 843–850 (2002).
Kontgen, F. et al. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes Dev. 9, 1965–1977 (1995).
Shinkura, R. et al. Alymphoplasia is caused by a point mutation in the mouse gene encoding Nf-κB-inducing kinase. Nat. Genet. 22, 74–77 (1999).
Novack, D.V. et al. The IκB function of NF-κB2 p100 controls stimulated osteoclastogenesis. J. Exp. Med. 198, 771–781 (2003).
Yin, L. et al. Defective lymphotoxin-β receptor-induced NF-κB transcriptional activity in NIK-deficient mice. Science 291, 2162–2165 (2001).
Fagarasan, S. et al. Alymphoplasia (aly)-type nuclear factor κB-inducing kinase (NIK) causes defects in secondary lymphoid tissue chemokine receptor signaling and homing of peritoneal cells to the gut-associated lymphatic tissue system. J. Exp. Med. 191, 1477–1486 (2000).
Yamada, T. et al. Abnormal immune function of hemopoietic cells from alymphoplasia (aly) mice, a natural strain with mutant NF-κB-inducing kinase. J. Immunol. 165, 804–812 (2000).
Kajiura, F. et al. NF-κB-inducing kinase establishes self-tolerance in a thymic stroma-dependent manner. J. Immunol. 172, 2067–2075 (2004).
Weih, F. et al. Both multiorgan inflammation and myeloid hyperplasia in RelB-deficient mice are T cell dependent. J. Immunol. 157, 3974–3979 (1996).
Tsubata, R. et al. Autoimmune disease of exocrine organs in immunodeficient alymphoplasia mice: a spontaneous model for Sjogren's syndrome. Eur. J. Immunol. 26, 2742–2748 (1996).
Dutton, R.W., Bradley, L.M. & Swain, S.L. T cell memory. Annu. Rev. Immunol. 16, 201–223 (1998).
Sprent, J. & Surh, C.D. T cell memory. Annu. Rev. Immunol. 20, 551–579 (2002).
Sakaguchi, S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22, 531–562 (2004).
Fehervari, Z. & Sakaguchi, S. Development and function of CD25+CD4+ regulatory T cells. Curr. Opin. Immunol. 16, 203–208 (2004).
Piccirillo, C.A. & Shevach, E.M. Naturally-occurring CD4+CD25+ immunoregulatory T cells: central players in the arena of peripheral tolerance. Semin. Immunol. 16, 81–88 (2004).
Khattri, R., Cox, T., Yasayko, S.A. & Ramsdell, F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4, 337–342 (2003).
Fontenot, J.D., Gavin, M.A. & Rudensky, A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4, 330–336 (2003).
Hori, S., Nomura, T. & Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061 (2003).
Lu, L.F., Gondek, D.C., Scott, Z.A. & Noelle, R.J. NF κB-inducing kinase deficiency results in the development of a subset of regulatory T cells, which shows a hyperproliferative activity upon glucocorticoid-induced TNF receptor family-related gene stimulation. J. Immunol. 175, 1651–1657 (2005).
Ernst, B., Lee, D-S., Chang, J., Sprent, J. & Surh, C.D. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity 11, 173–181 (1999).
Weil, R. & Israel, A. T-cell-receptor- and B-cell-receptor-mediated activation of NF-κB in lymphocytes. Curr. Opin. Immunol. 16, 374–381 (2004).
Kim, H.P. & Leonard, W.J. The basis for TCR-mediated regulation of the IL-2 receptor α chain gene: role of widely separated regulatory elements. EMBO J. 21, 3051–3059 (2002).
Schuh, K. et al. The interleukin 2 receptor α chain/CD25 promoter is a target for nuclear factor of activated T cells. J. Exp. Med. 188, 1369–1373 (1998).
Bren, G.D. et al. Transcription of the RelB gene is regulated by NF-κB. Oncogene 20, 7722–7733 (2001).
Olashaw, N.E. Inducible activation of RelB in fibroblasts. J. Biol. Chem. 271, 30307–30310 (1996).
Ivanov, V.N., Deng, G., Podack, E.R. & Malek, T.R. Pleiotropic effects of Bcl-2 on transcription factors in T cells: potential role of NF-κB p50-p50 for the anti-apoptotic function of Bcl-2. Int. Immunol. 7, 1709–1720 (1995).
Robertson, J.M., Jensen, P.E. & Evavold, B.D. DO11.10 and OT-II T cells recognize a C-terminal ovalbumin 323–339 epitope. J. Immunol. 164, 4706–4712 (2000).
Kishimoto, H. & Sprent, J. Strong TCR ligation without costimulation causes rapid onset of Fas-dependent apoptosis of naive murine CD4+ T cells. J. Immunol. 163, 1817–1826 (1999).
Assenmacher, M., Schmitz, J. & Radbruch, A. Flow cytometric determination of cytokines in activated murine T helper lymphocytes: expression of interleukin-10 in interferon-γ and in interleukin-4-expressing cells. Eur. J. Immunol. 24, 1097–1101 (1994).
Grundstrom, S., Anderson, P., Scheipers, P. & Sundstedt, A. Bcl-3 and NFκB p50-p50 homodimers act as transcriptional repressors in tolerant CD4+ T cells. J. Biol. Chem. 279, 8460–8468 (2004).
Zal, T. & Gascoigne, N.R. Using live FRET imaging to reveal early protein-protein interactions during T cell activation. Curr. Opin. Immunol. 16, 418–427 (2004).
Ishimaru, N. et al. Development of autoimmune exocrinopathy resembling Sjogren's syndrome in estrogen-deficient mice of healthy background. Am. J. Pathol. 163, 1481–1490 (2003).
Acknowledgements
We thank B. Marchand for typing the manuscript. Supported by the United States Public Health Service (CA38355, AI21487, AI46710 and AG01743; publication number 17327-IMM from The Scripps Research Institute) and by the Ministry of Education, Science, and Culture of Japan (grants-in-aid for scientific research; 17689049).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Proliferation of T cell subsets. (PDF 428 kb)
Supplementary Fig. 2
Suppressive effect of aly/aly memory CD4+ T cells. (PDF 214 kb)
Supplementary Fig. 3
Expression of Foxp3 in CD4+ cell subsets. (PDF 92 kb)
Supplementary Fig. 4
Comparison of memory CD4+ and CD25+CD4+ cells. (PDF 183 kb)
Supplementary Fig. 5
NF-κB1/NF-κB2 association. (PDF 185 kb)
Supplementary Fig. 6
Immunoblots for NF-κB subunits in Jurkat or A431 cell lysates. (PDF 71 kb)
Rights and permissions
About this article
Cite this article
Ishimaru, N., Kishimoto, H., Hayashi, Y. et al. Regulation of naive T cell function by the NF-κB2 pathway. Nat Immunol 7, 763–772 (2006). https://doi.org/10.1038/ni1351
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1351
This article is cited by
-
NF-κB in control of regulatory T cell development, identity, and function
Journal of Molecular Medicine (2022)
-
Biological characteristics of transcription factor RelB in different immune cell types: implications for the treatment of multiple sclerosis
Molecular Brain (2019)
-
Role of NF-kappaB2-p100 in regulatory T cell homeostasis and activation
Scientific Reports (2019)
-
TNFR2 ligation in human T regulatory cells enhances IL2-induced cell proliferation through the non-canonical NF-κB pathway
Scientific Reports (2018)
-
Impact of loss of NF‐κB1, NF‐κB2 or c‐REL on SLE‐like autoimmune disease and lymphadenopathy in Faslpr/lpr mutant mice
Immunology & Cell Biology (2016)