Abstract
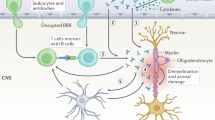
Understanding of autoimmune diseases, including multiple sclerosis, has expanded considerably in recent years. New insights have been provided by not only animal models but also studies of patients, often in conjunction with experimental therapies. It is accepted that autoimmune T cells mediate the early steps of new multiple sclerosis lesions, and although uncertainties remain about the specific targets of autoreactive T cells, several studies indicate myelin antigens. Recent findings obtained with both animal models and patients with multiple sclerosis indicate involvement of a T helper cell with a TH-17 phenotype, in contrast to previous data indicating that T helper type 1 cells are critical. Evidence has also been presented for CD8+ and regulatory T cell populations, although their involvement remains to be established. Despite evidence supporting the idea that autoreactive T cells are involved in disease induction, cells of myeloid lineage, antibodies and complement as well as processes intrinsic to the central nervous system seem to determine the effector stages of tissue damage. Careful analysis of the alterations in immune processes should further advance knowledge of the relationship between the inflammatory component of this disease and the more diffuse degeneration of progressive multiple sclerosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Stone, L.A. et al. Blood-brain barrier disruption on contrast-enhanced MRI in patients with mild relapsing-remitting multiple sclerosis: relationship to course, gender, and age. Neurology 45, 1122–1126 (1995).
Coles, A.J. et al. Monoclonal antibody treatment exposes three mechanisms underlying the clinical course of multiple sclerosis. Ann. Neurol. 46, 296–304 (1999).
Hartung, H.P. et al. Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet 360, 2018–2025 (2002).
Oksenberg, J.R. & Hauser, S.L. Genetics of multiple sclerosis. Neurol. Clin. 23, 61–75 (2005).
McKenzie, B.S., Kastelein, R.A. & Cua, D.J. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 27, 17–23 (2006).
Iwakura, Y. & Ishigame, H. The IL-23/IL-17 axis in inflammation. J. Clin. Invest. 116, 1218–1222 (2006).
Brok, H.P. et al. Prevention of experimental autoimmune encephalomyelitis in common marmosets using an anti-IL-12p40 monoclonal antibody. J. Immunol. 169, 6554–6563 (2002).
Cua, D.J. et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421, 744–748 (2003).
Veldhoen, M., Hocking, R.J., Atkins, C.J., Locksley, R.M. & Stockinger, B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189 (2006).
Gijbels, K., Brocke, S., Abrams, J.S. & Steinman, L. Administration of neutralizing antibodies to interleukin-6 (IL-6) reduces experimental autoimmune encephalomyelitis and is associated with elevated levels of IL-6 bioactivity in central nervous system and circulation. Mol. Med. 1, 795–805 (1995).
Korn, T. et al. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature 448, 484–487 (2007).
Nurieva, R. et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 448, 480–483 (2007).
Wilson, N.J. et al. Development, cytokine profile and function of human interleukin 17–producing helper T cells. Nat. Immunol. 8, 950–957 (2007).
Acosta-Rodriguez, E.V. et al. Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17–producing human T helper cells Nat. Immunol. 8, 942–949 (2007).
Ivanov, I.I. et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006).
Liao, J.J., Huang, M.C. & Goetzl, E.J. Cutting edge: alternative signaling of TH17 cell development by sphingosine 1-phosphate. J. Immunol. 178, 5425–5428 (2007).
Kappos, L. et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N. Engl. J. Med. 355, 1124–1140 (2006).
Matusevicius, D. et al. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult. Scler. 5, 101–104 (1999).
Lock, C. et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat. Med. 8, 500–508 (2002).
Vaknin-Dembinsky, A., Balashov, K. & Weiner, H.L. IL-23 is increased in dendritic cells in multiple sclerosis and down-regulation of IL-23 by antisense oligos increases dendritic cell IL-10 production. J. Immunol. 176, 7768–7774 (2006).
Li, Y. et al. Increased IL-23p19 expression in multiple sclerosis lesions and its induction in microglia. Brain 130, 490–501 (2007).
Traugott, U., Reinherz, E.L. & Raine, C.S. Multiple sclerosis: distribution of T cell subsets within active chronic lesions. Science 219, 308–310 (1983).
Crawford, M.P. et al. High prevalence of autoreactive, neuroantigen-specific CD8+ T cells in multiple sclerosis revealed by novel flow cytometric assay. Blood 103, 4222–4231 (2004).
Babbe, H. et al. Clonal expansions of CD8+ T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J. Exp. Med. 192, 393–404 (2000).
Ji, Q. & Goverman, J. Experimental autoimmune encephalomyelitis mediated by CD8+ T cells. Ann. NY Acad. Sci. 2203, 157–166 (2007).
Brisebois, M., Zehntner, S.P., Estrada, J., Owens, T. & Fournier, S. A pathogenic role for CD8+ T cells in a spontaneous model of demyelinating disease. J. Immunol. 177, 2403–2411 (2006).
Medana, I., Martinic, M.A., Wekerle, H. & Neumann, H. Transection of major histocompatibility complex class I-induced neurites by cytotoxic T lymphocytes. Am. J. Pathol. 159, 809–815 (2001).
Coles, A.J. & Compston, A. Efficiency of alemtuzumab in treatment-naive relapsing remiting multiple sclerosis: analysis after two years of study. Neurology 68, A100 (2007).
Saccardi, R. et al. Autologous stem cell transplantation for progressive multiple sclerosis: update of the European Group for Blood and Marrow Transplantation autoimmune diseases working party database. Mult. Scler. 12, 814–823 (2006).
Muraro, P.A. et al. Thymic output generates a new and diverse TCR repertoire after autologous stem cell transplantation in multiple sclerosis patients. J. Exp. Med. 201, 805–816 (2005).
Bailey, S.L., Schreiner, B. & McMahon, E.J. CNS myeloid DCs presenting endogenous myelin peptides 'preferentially' polarize CD4+ TH-17 cells in relapsing EAE. Nat. Immunol. 8, 172–180 (2007).
Bailey, S.L., Schreiner, B., McMahon, E.J. & Miller, S.D. CNS myeloid DCs presenting endogenous myelin peptides 'preferentially' polarize CD4+ TH-17 cells in relapsing EAE. Nat. Immunol. 8, 172–180 (2007).
Hauser, S.L. et al. A phase II randomized, placebo-controlled trial of rituximab in adult with relapsing remitting multiple sclerosis. Neurology 68, A99 (2007).
Sospedra, M. & Martin, R. Immunology of multiple sclerosis. Annu. Rev. Immunol. 23, 683–747 (2005).
Bielekova, B. et al. Expansion and functional relevance of high-avidity myelin-specific CD4+ T cells in multiple sclerosis. J. Immunol. 172, 3893–3904 (2004).
Bielekova, B. et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat. Med. 6, 1167–1175 (2000).
Muraro, P.A. et al. Molecular tracking of antigen-specific T cell clones in neurological immune-mediated disorders. Brain 126, 20–31 (2003).
Fujinami, R.S., von Herrath, M.G., Christen, U. & Whitton, J.L. Molecular mimicry, bystander activation, or viral persistence: infections and autoimmune disease. Clin. Microbiol. Rev. 19, 80–94 (2006).
Marshak-Rothstein, A. & Rifkin, I.R. Immunologically active autoantigens: the role of toll-like receptors in the development of chronic inflammatory disease. Annu. Rev. Immunol. 25, 419–441 (2007).
Madsen, L.S. et al. A humanized model for multiple sclerosis using HLA-DR2 and a human T-cell receptor. Nat. Genet. 23, 343–347 (1999).
Ellmerich, S. et al. High incidence of spontaneous disease in an HLA-DR15 and TCR transgenic multiple sclerosis model. J. Immunol. 174, 1938–1946 (2005).
Ito, K. et al. HLA-DR4-IE chimeric class II transgenic, murine class II-deficient mice are susceptible to experimental allergic encephalomyelitis. J. Exp. Med. 183, 2635–2644 (1996).
Huh, J. et al. Limited repertoire of HLA-DRB1*0401-restricted MBP111–129-specific T cells in HLA-DRB1*0401 Tg mice and their pathogenic potential. J. Neuroimmunol. 151, 94–102 (2004).
Quandt, J.A. et al. Unique clinical and pathological features in HLA-DRB1*0401-restricted MBP 111–129-specific humanized TCR transgenic mice. J. Exp. Med. 200, 223–234 (2004).
Ousman, S.S. et al. Protective and therapeutic role for αB-crystallin in autoimmune demyelination. Nature 48, 474–479 (2007).
Vanderlugt, C.L. et al. Pathologic role and temporal appearance of newly emerging autoepitopes in relapsing experimental autoimmune encephalomyelitis. J. Immunol. 164, 670–678 (2000).
McRae, B.L., Vanderlugt, C.L., Dal Canto, M.C. & Miller, S.D. Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J. Exp. Med. 182, 75–85 (1995).
Filippi, M. et al. Quantitative brain MRI lesion load predicts the course of clinically isolated syndromes suggestive of multiple sclerosis. Neurology 44, 635–641 (1994).
Ho, P.P. et al. A suppressive oligodeoxynucleotide enhances the efficacy of myelin cocktail/IL-4-tolerizing DNA vaccination and treats autoimmune disease. J. Immunol. 175, 6226–6234 (2005).
Stone, L.A. et al. The effect of interferon-β on blood-brain barrier disruptions demonstrated by contrast-enhanced magnetic resonance imaging in relapsing-remitting multiple sclerosis. Ann. Neurol. 37, 611–619 (1995).
Li, D.K., Zhao, G.J. & Paty, D.W. Randomized controlled trial of interferon-β-1a in secondary progressive MS: MRI results. Neurology 56, 1505–1513 (2001).
Calabresi, P.A. et al. Increases in soluble VCAM-1 correlate with a decrease in MRI lesions in multiple sclerosis treated with interferon β-1b. Ann. Neurol. 41, 669–674 (1997).
Polman, C.H. et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 354, 899–910 (2006).
Stuve, O. et al. Interferon β-1b decreases the migration of T lymphocytes in vitro: effects on matrix metalloproteinase-9. Ann. Neurol. 40, 853–863 (1996).
Avolio, C. et al. Serum MMP-9/TIMP-1 and MMP-2/TIMP-2 ratios in multiple sclerosis: relationships with different magnetic resonance imaging measures of disease activity during IFN-β-1a treatment. Mult. Scler. 11, 441–446 (2005).
Argaw, A.T. et al. IL-1β regulates blood-brain barrier permeability via reactivation of the hypoxia-angiogenesis program. J. Immunol. 177, 5574–5584 (2006).
Farina, C. et al. Preferential expression and function of Toll-like receptor 3 in human astrocytes. J. Neuroimmunol. 159, 12–19 (2005).
Kohm, A.P., Carpentier, P.A., Anger, H.A. & Miller, S.D. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J. Immunol. 169, 4712–4716 (2002).
Cabbage, S.E. et al. Regulatory T cells maintain long-term tolerance to myelin basic protein by inducing a novel, dynamic state of T cell tolerance. J. Immunol. 178, 887–896 (2007).
Walker, M.R. et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25− T cells. J. Clin. Invest. 112, 1437–1443 (2003).
von Boehmer, H. Mechanisms of suppression by suppressor T cells. Nat. Immunol. 6, 338–344 (2005).
Korn, T. et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat. Med. 13, 423–431 (2007).
Bielekova, B. et al. Humanized anti-CD25 (daclizumab) inhibits disease activity in multiple sclerosis patients failing to respond to interferon β. Proc. Natl. Acad. Sci. USA 101, 8705–8708 (2004).
Kreijveld, E., Koenen, H.J., Klasen, I.S., Hilbrands, L.B. & Joosten, I. Following anti-CD25 treatment, a functional CD4+CD25+ regulatory T-cell pool is present in renal transplant recipients. Am. J. Transplant. 7, 249–255 (2007).
Bielekova, B. et al. Regulatory CD56bright natural killer cells mediate immunomodulatory effects of IL-2Rα-targeted therapy (daclizumab) in multiple sclerosis. Proc. Natl. Acad. Sci. USA 103, 5941–5946 (2006).
Julia, E. et al. Deficient Fas expression by CD4+CCR5+ T cells in multiple sclerosis. J. Neuroimmunol. 180, 147–158 (2006).
Tennakoon, D.K. et al. Therapeutic induction of regulatory, cytotoxic CD8+ T cells in multiple sclerosis. J. Immunol. 176, 7119–7129 (2006).
Hur, E.M. et al. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nat. Immunol. 8, 74–83 (2007).
Chabas, D. et al. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science 294, 1731–1735 (2001).
Meller, R. et al. Neuroprotection by osteopontin in stroke. J. Cereb. Blood Flow Metab. 25, 217–225 (2005).
Platten, M. et al. Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science 310, 850–855 (2005).
Sakurai, K., Zou, J.P., Tschetter, J.R., Ward, J.M. & Shearer, G.M. Effect of indoleamine 2,3-dioxygenase on induction of experimental autoimmune encephalomyelitis. J. Neuroimmunol. 129, 186–196 (2002).
Vollmer, T. et al. Oral simvastatin treatment in relapsing-remitting multiple sclerosis. Lancet 363, 1607–1608 (2004).
Youssef, S. et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature 420, 78–84 (2002).
van Walderveen, M.A. et al. Histopathologic correlate of hypointense lesions on T1-weighted spin-echo MRI in multiple sclerosis. Neurology 50, 1282–1288 (1998).
Bagnato, F. et al. Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain 126, 1782–1789 (2003).
Rebenko-Moll, N.M., Liu, L., Cardona, A. & Ransohoff, R.M. Chemokines, mononuclear cells and the nervous system: heaven (or hell) is in the details. Curr. Opin. Immunol. 18, 683–689 (2006).
Furtado, G.C. et al. A novel model of demyelinating encephalomyelitis induced by monocytes and dendritic cells. J. Immunol. 177, 6871–6879 (2006).
Lucchinetti, C. et al. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann. Neurol. 47, 707–717 (2000).
Magliozzi, R. et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 130, 1089–1104 (2007).
Cepok, S. et al. Identification of Epstein-Barr virus proteins as putative targets of the immune response in multiple sclerosis. J. Clin. Invest. 115, 1352–1360 (2005).
Genain, C.P. et al. Antibody facilitation of multiple sclerosis-like lesions in a nonhuman primate. J. Clin. Invest. 96, 2966–2974 (1995).
Berger, T. et al. Antimyelin antibodies as a predictor of clinically definite multiple sclerosis after a first demyelinating event. N. Engl. J. Med. 349, 139–145 (2003).
Kuhle, J. et al. Lack of association between antimyelin antibodies and progression to multiple sclerosis. N. Engl. J. Med. 356, 371–378 (2007).
Allen, I.V., McQuaid, S., Mirakhur, M. & Nevin, G. Pathological abnormalities in the normal-appearing white matter in multiple sclerosis. Neurol. Sci. 22, 141–144 (2001).
Ponomarev, E.D., Shriver, L.P., Maresz, K. & Dittel, B.N. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J. Neurosci. Res. 81, 374–389 (2005).
Pitt, D., Werner, P. & Raine, C.S. Glutamate excitotoxicity in a model of multiple sclerosis. Nat. Med. 6, 67–70 (2000).
Killestein, J., Kalkers, N.F. & Polman, C.H. Glutamate inhibition in MS: the neuroprotective properties of riluzole. J. Neurol. Sci. 233, 113–115 (2005).
Acknowledgements
Supported by the Gemeinnützige Hertie Stiftung (R.M.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
McFarland, H., Martin, R. Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol 8, 913–919 (2007). https://doi.org/10.1038/ni1507
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1507
This article is cited by
-
The gateway reflex regulates tissue-specific autoimmune diseases
Inflammation and Regeneration (2024)
-
Causal role of immune cells in schizophrenia: Mendelian randomization (MR) study
BMC Psychiatry (2023)
-
Teriflunomide Concentrations in Cerebrospinal Fluid and Plasma in Patients with Multiple Sclerosis: A Pharmacokinetic Study
CNS Drugs (2023)
-
Tissue-resident immune cells in the pathogenesis of multiple sclerosis
Inflammation Research (2023)
-
The importance of assessing sleep disorders in multiple sclerosis
Sleep and Breathing (2023)