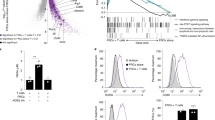
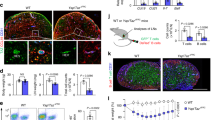
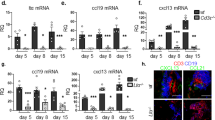
Abstract
Interleukin 7 is essential for the survival of naive T lymphocytes. Despite its importance, its cellular source in the periphery remains poorly defined. Here we report a critical function for lymph node access in T cell homeostasis and identify T zone fibroblastic reticular cells in these organs as the main source of interleukin 7. In vitro, T zone fibroblastic reticular cells were able to prevent the death of naive T lymphocytes but not of B lymphocytes by secreting interleukin 7 and the CCR7 ligand CCL19. Using gene-targeted mice, we demonstrate a nonredundant function for CCL19 in T cell homeostasis. Our data suggest that lymph nodes and T zone fibroblastic reticular cells have a key function in naive CD4+ and CD8+ T cell homeostasis by providing a limited reservoir of survival factors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Marrack, P. & Kappler, J. Control of T cell viability. Annu. Rev. Immunol. 22, 765–787 (2004).
Surh, C.D. & Sprent, J. Regulation of mature T cell homeostasis. Semin. Immunol. 17, 183–191 (2005).
Freitas, A.A. & Rocha, B. Population biology of lymphocytes: the flight for survival. Annu. Rev. Immunol. 18, 83–111 (2000).
Jiang, Q. et al. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 16, 513–533 (2005).
Trinder, P.K. & Maeurer, M.J. in The Cytokine Handbook (eds. Thomson, A.W. & Lotze, M.T.) 305–345 (Elsevier Science, London, 2003).
Ma, A., Koka, R. & Burkett, P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu. Rev. Immunol. 24, 657–679 (2006).
Cinalli, R.M. et al. T cell homeostasis requires G protein-coupled receptor-mediated access to trophic signals that promote growth and inhibit chemotaxis. Eur. J. Immunol. 35, 786–795 (2005).
Dai, Z. & Lakkis, F.G. Cutting edge: Secondary lymphoid organs are essential for maintaining the CD4, but not CD8, naive T cell pool. J. Immunol. 167, 6711–6715 (2001).
Dummer, W., Ernst, B., LeRoy, E., Lee, D. & Surh, C. Autologous regulation of naive T cell homeostasis within the T cell compartment. J. Immunol. 166, 2460–2468 (2001).
Cyster, J.G. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu. Rev. Immunol. 23, 127–159 (2005).
von Andrian, U.H. & Mempel, T.R. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 3, 867–878 (2003).
Bajenoff, M. et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity 25, 989–1001 (2006).
Gretz, J.E., Anderson, A.O. & Shaw, S. Cords, channels, corridors and conduits: critical architectural elements facilitating cell interactions in the lymph node cortex. Immunol. Rev. 156, 11–24 (1997).
Katakai, T., Hara, T., Sugai, M., Gonda, H. & Shimizu, A. Lymph node fibroblastic reticular cells construct the stromal reticulum via contact with lymphocytes. J. Exp. Med. 200, 783–795 (2004).
Luther, S.A., Tang, H.L., Hyman, P.L., Farr, A.G. & Cyster, J.G. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc. Natl. Acad. Sci. USA 97, 12694–12699 (2000).
Lepault, F., Gagnerault, M.C., Faveeuw, C. & Boitard, C. Recirculation, phenotype and functions of lymphocytes in mice treated with monoclonal antibody MEL-14. Eur. J. Immunol. 24, 3106–3112 (1994).
Lo, C.G., Xu, Y., Proia, R.L. & Cyster, J.G. Cyclical modulation of sphingosine-1-phosphate receptor 1 surface expression during lymphocyte recirculation and relationship to lymphoid organ transit. J. Exp. Med. 201, 291–301 (2005).
Lo, C.G., Lu, T.T. & Cyster, J.G. Integrin-dependence of lymphocyte entry into the splenic white pulp. J. Exp. Med. 197, 353–361 (2003).
Berlin-Rufenach, C. et al. Lymphocyte migration in lymphocyte function-associated antigen (LFA)-1-deficient mice. J. Exp. Med. 189, 1467–1478 (1999).
Nakano, H. et al. A novel mutant gene involved in T-lymphocyte-specific homing into peripheral lymphoid organs on mouse chromosome 4. Blood 91, 2886–2895 (1998).
Gunn, M.D. et al. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J. Exp. Med. 189, 451–460 (1999).
Farr, A.G. et al. Characterization and cloning of a novel glycoprotein expressed by stromal cells in T-dependent areas of peripheral lymphoid tissues. J. Exp. Med. 176, 1477–1482 (1992).
Kriehuber, E. et al. Isolation and characterization of dermal lymphatic and blood endothelial cells reveal stable and functionally specialized cell lineages. J. Exp. Med. 194, 797–808 (2001).
Honda, K. et al. Molecular basis for hematopoietic/mesenchymal interaction during initiation of Peyer's patch organogenesis. J. Exp. Med. 193, 621–630 (2001).
Sixt, M. et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity 22, 19–29 (2005).
Ngo, V.N. et al. Lymphotoxin alpha/beta and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J. Exp. Med. 189, 403–412 (1999).
Gretz, J.E., Norbury, C.C., Anderson, A.O., Proudfoot, A.E. & Shaw, S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J. Exp. Med. 192, 1425–1440 (2000).
Bergers, G. & Song, S. The role of pericytes in blood-vessel formation and maintenance. Neuro. Oncol. 7, 452–464 (2005).
Mazzucchelli, R. & Durum, S.K. Interleukin-7 receptor expression: intelligent design. Nat. Rev. Immunol. 7, 144–154 (2007).
Hinz, B. & Gabbiani, G. Mechanisms of force generation and transmission by myofibroblasts. Curr. Opin. Biotechnol. 14, 538–546 (2003).
Gabbiani, G. The myofibroblast in wound healing and fibrocontractive diseases. J. Pathol. 200, 500–503 (2003).
Hinz, B., Celetta, G., Tomasek, J.J., Gabbiani, G. & Chaponnier, C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol. Biol. Cell 12, 2730–2741 (2001).
Sanchez-Sanchez, N. et al. Chemokine receptor CCR7 induces intracellular signaling that inhibits apoptosis of mature dendritic cells. Blood 104, 619–625 (2004).
Endharti, A.T., Zhou, Y.W., Nakashima, I. & Suzuki, H. Galectin-1 supports survival of naive T cells without promoting cell proliferation. Eur. J. Immunol. 35, 86–97 (2005).
Kimura, K. et al. Role of glycosaminoglycans in the regulation of T cell proliferation induced by thymic stroma-derived T cell growth factor. J. Immunol. 146, 2618–2624 (1991).
Ueno, T. et al. Role for CCR7 ligands in the emigration of newly generated T lymphocytes from the neonatal thymus. Immunity 16, 205–218 (2002).
Mackay, F. & Ambrose, C. The TNF family members BAFF and APRIL: the growing complexity. Cytokine Growth Factor Rev. 14, 311–324 (2003).
Schluns, K.S., Kieper, W.C., Jameson, S.C. & Lefrancois, L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 1, 426–432 (2000).
Zhou, Y.W. et al. Murine lymph node-derived stromal cells effectively support survival but induce no activation/proliferation of peripheral resting T cells in vitro. Immunology 109, 496–503 (2003).
Feuillet, V., Lucas, B., Di Santo, J.P., Bismuth, G. & Trautmann, A. Multiple survival signals are delivered by dendritic cells to naive CD4+ T cells. Eur. J. Immunol. 35, 2563–2572 (2005).
Hara, T. et al. A transmembrane chemokine, CXC chemokine ligand 16, expressed by lymph node fibroblastic reticular cells has the potential to regulate T cell migration and adhesion. Int. Immunol. 18, 301–311 (2006).
Forster, R. et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99, 23–33 (1999).
Luther, S.A. et al. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J. Immunol. 169, 424–433 (2002).
Ploix, C., Lo, D. & Carson, M.J. A ligand for the chemokine receptor CCR7 can influence the homeostatic proliferation of CD4 T cells and progression of autoimmunity. J. Immunol. 167, 6724–6730 (2001).
Kim, J.W., Ferris, R.L. & Whiteside, T.L. Chemokine C receptor 7 expression and protection of circulating CD8+ T lymphocytes from apoptosis. Clin. Cancer Res. 11, 7901–7910 (2005).
Sanchez-Sanchez, N., Riol-Blanco, L. & Rodriguez-Fernandez, J.L. The multiple personalities of the chemokine receptor CCR7 in dendritic cells. J. Immunol. 176, 5153–5159 (2006).
Okada, T. & Cyster, J.G. CC chemokine receptor 7 contributes to Gi-dependent T cell motility in the lymph node. J. Immunol. 178, 2973–2978 (2007).
Worbs, T., Mempel, T.R., Bolter, J., von Andrian, U.H. & Forster, R. CCR7 ligands stimulate the intranodal motility of T lymphocytes in vivo. J. Exp. Med. 204, 489–495 (2007).
Zamisch, M. et al. Ontogeny and regulation of IL-7-expressing thymic epithelial cells. J. Immunol. 174, 60–67 (2005).
Goffin, J.M. et al. Focal adhesion size controls tension-dependent recruitment of α-smooth muscle actin to stress fibers. J. Cell Biol. 172, 259–268 (2006).
Acknowledgements
We thank M. Ansel, S. Bell, M. Charmoy, T. Andresen, P. Hyman, N. Killeen and J. Smith-Clerc for technical help; H. Robson MacDonald for critical reading of the manuscript; M. Cooper, W. van Ewijk, A. Farr, G. Gabbiani, B. Imhof, C. Ruegg and A. Wilson for antibodies; and N. Killeen for embryonic stem cells. Supported by the Swiss National Science Foundation (PPOOA-68805 to S.A.L.), the Boehringer Ingelheim Fonds (T.K.V.), the Swiss National Science Foundation (3100A0-102150/1 and 3100A0-113733/1 to B.H.) and the National Institutes of Health (AI45073 to J.G.C.).
Author information
Authors and Affiliations
Contributions
A.L. did the in vivo experiments, with assistance from M.R.B. and S.F., as well as all the in vitro assays, flow cytometry sorting and analysis, and in situ hybridization, and contributed to the writing of the manuscript; T.K.V. did the wrinkling assays in collaboration with B.H. and did the RT-PCR and assisted with the flow cytometry sorting and analysis; S.F. did most of the immunofluorescence with assistance from T.K.V.; H.A.-O. provided antibodies; S.A.L. generated the CCL19-deficient mice in the laboratory of J.G.C.; S.A.L. directed the study and wrote the manuscript; and all authors critically reviewed the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 and Tables 1–4 (PDF 2774 kb)
Rights and permissions
About this article
Cite this article
Link, A., Vogt, T., Favre, S. et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol 8, 1255–1265 (2007). https://doi.org/10.1038/ni1513
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1513
This article is cited by
-
Fibroblast and myofibroblast activation in normal tissue repair and fibrosis
Nature Reviews Molecular Cell Biology (2024)
-
Splenic stromal niches in homeostasis and immunity
Nature Reviews Immunology (2023)
-
Cancer-associated fibroblast classification in single-cell and spatial proteomics data
Nature Communications (2023)
-
Patient-derived xenograft models in cancer therapy: technologies and applications
Signal Transduction and Targeted Therapy (2023)
-
Soluble factors from TLR4- or TCR-activated cells contribute to stability of the resting phenotype and increase the expression of CXCR4 of human memory CD4 T cells
Immunologic Research (2023)