Abstract
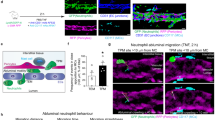
Selectins and their ligands mediate leukocyte rolling, allowing interactions with chemokines that lead to integrin activation and arrest. Here we show that E-selectin is crucial for generating a secondary wave of activating signals, transduced specifically by E-selectin ligand-1, that induces polarized, activated αMβ2 integrin clusters at the leading edge of crawling neutrophils, allowing capture of circulating erythrocytes or platelets. In a humanized mouse model of sickle cell disease, the capture of erythrocytes by αMβ2 microdomains leads to acute lethal vascular occlusions. In a model of transfusion-related acute lung injury, polarized neutrophils capture circulating platelets, resulting in the generation of oxidative species that produce vascular damage and lung injury. Inactivation of E-selectin or αMβ2 prevents tissue injury in both inflammatory models, suggesting broad implications of this paradigm in thromboinflammatory diseases. These results indicate that endothelial selectins can influence neutrophil behavior beyond its canonical rolling step through delayed, organ-damaging, polarized activation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bullard, D.C. et al. Infectious susceptibility and severe deficiency of leukocyte rolling and recruitment in E-selectin and P-selectin double mutant mice. J. Exp. Med. 183, 2329–2336 (1996).
Frenette, P.S., Mayadas, T.N., Rayburn, H., Hynes, R.O. & Wagner, D.D. Susceptibility to infection and altered hematopoiesis in mice deficient in both P-and E-selectins. Cell 84, 563–574 (1996).
Labow, M.A. et al. Characterization of E-selectin-deficient mice: demonstration of overlapping function of the endothelial selectins. Immunity 1, 709–720 (1994).
Hidalgo, A., Peired, A.J., Wild, M.K., Vestweber, D. & Frenette, P.S. Complete identification of E-selectin ligands on neutrophils reveals distinct functions of PSGL-1, ESL-1, and CD44. Immunity 26, 477–489 (2007).
Zarbock, A., Lowell, C.A. & Ley, K. Spleen tyrosine kinase Syk is necessary for E-selectin-induced αLβ2 integrin–mediated rolling on intercellular adhesion molecule-1. Immunity 26, 773–783 (2007).
Steegmaler, M. et al. The E-selectin-ligand ESL-1 is a variant of a receptor for fibroblast growth factor. Nature 373, 615–620 (1995).
Lo, S.K. et al. Endothelial-leukocyte adhesion molecule 1 stimulates the adhesive activity of leukocyte integrin CR3 (CD11b/CD18, Mac-1, αMβ2) on human neutrophils. J. Exp. Med. 173, 1493–1500 (1991).
Simon, S.I., Hu, Y., Vestweber, D. & Smith, C.W. Neutrophil tethering on E-selectin activates β2 integrin binding to ICAM-1 through a mitogen-activated protein kinase signal transduction pathway. J. Immunol. 164, 4348–4358 (2000).
Gahmberg, C.G., Tolvanen, M. & Kotovuori, P. Leukocyte adhesion—structure and function of human leukocyte β2-integrins and their cellular ligands. Eur. J. Biochem. 245, 215–232 (1997).
Beller, D.I., Springer, T.A. & Schreiber, R.D. Anti–Mac-1 selectively inhibits the mouse and human type three complement receptor. J. Exp. Med. 156, 1000–1009 (1982).
Simon, D.I. et al. Platelet glycoprotein ibα is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18). J. Exp. Med. 192, 193–204 (2000).
Barreiro, O., de la Fuente, H., Mittelbrunn, M. & Sanchez-Madrid, F. Functional insights on the polarized redistribution of leukocyte integrins and their ligands during leukocyte migration and immune interactions. Immunol. Rev. 218, 147–164 (2007).
Tohyama, Y. et al. The critical cytoplasmic regions of the αL/β2 integrin in Rap1-induced adhesion and migration. Mol. Biol. Cell 14, 2570–2582 (2003).
Ridley, A.J. et al. Cell migration: integrating signals from front to back. Science 302, 1704–1709 (2003).
Abboud, M., Laver, J. & Blau, C.A. Granulocytosis causing sickle-cell crisis. Lancet 351, 959 (1998).
Hirahashi, J. et al. Mac-1 signaling via Src-family and Syk kinases results in elastase-dependent thrombohemorrhagic vasculopathy. Immunity 25, 271–283 (2006).
Looney, M.R., Su, X., Van Ziffle, J.A., Lowell, C.A. & Matthay, M.A. Neutrophils and their Fcγ receptors are essential in a mouse model of transfusion-related acute lung injury. J. Clin. Invest. 116, 1615–1623 (2006).
Frenette, P.S. & Atweh, G.F. Sickle cell disease: old discoveries, new concepts, and future promise. J. Clin. Invest. 117, 850–858 (2007).
Stuart, M.J. & Nagel, R.L. Sickle-cell disease. Lancet 364, 1343–1360 (2004).
Chang, J., Shi, P.A., Chiang, E.Y. & Frenette, P.S. Intravenous immunoglobulins reverse acute vaso-occlusive crises in sickle cell mice through rapid inhibition of neutrophil adhesion. Blood 111, 915–923 (2008).
Turhan, A., Weiss, L.A., Mohandas, N., Coller, B.S. & Frenette, P.S. Primary role for adherent leukocytes in sickle cell vascular occlusion: a new paradigm. Proc. Natl. Acad. Sci. USA 99, 3047–3051 (2002).
Holness, L., Knippen, M.A., Simmons, L. & Lachenbruch, P.A. Fatalities caused by TRALI. Transfus. Med. Rev. 18, 184–188 (2004).
Silliman, C.C., Ambruso, D.R. & Boshkov, L.K. Transfusion-related acute lung injury. Blood 105, 2266–2273 (2005).
Falati, S., Gross, P., Merrill-Skoloff, G., Furie, B.C. & Furie, B. Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nat. Med. 8, 1175–1181 (2002).
Chiang, E.Y., Hidalgo, A., Chang, J. & Frenette, P.S. Imaging receptor microdomains on leukocyte subsets in live mice. Nat. Methods 4, 219–222 (2007).
Totani, L. et al. Src-family kinases mediate an outside-in signal necessary for β2 integrins to achieve full activation and sustain firm adhesion of polymorphonuclear leucocytes tethered on E-selectin. Biochem. J. 396, 89–98 (2006).
Smith, M.L., Olson, T.S. & Ley, K. CXCR2- and E-selectin–induced neutrophil arrest during inflammation in vivo. J. Exp. Med. 200, 935–939 (2004).
Simon, S.I. et al. L-selectin (CD62L) cross-linking signals neutrophil adhesive functions via the Mac-1 (CD11b/CD18) β2-integrin. J. Immunol. 155, 1502–1514 (1995).
Butcher, E.C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell 67, 1033–1036 (1991).
Lawrence, M.B. & Springer, T.A. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell 65, 859–873 (1991).
Lorant, D.E. et al. Inflammatory roles of P-selectin. J. Clin. Invest. 92, 559–570 (1993).
Evangelista, V. et al. Platelet/polymorphonuclear leukocyte interaction: P-selectin triggers protein-tyrosine phosphorylation-dependent CD11b/CD18 adhesion: role of PSGL-1 as a signaling molecule. Blood 93, 876–885 (1999).
Wang, H.B. et al. P-selectin primes leukocyte integrin activation during inflammation. Nat. Immunol. 8, 882–892 (2007).
Shappell, S.B. et al. Mac-1 (CD11b/CD18) mediates adherence-dependent hydrogen peroxide production by human and canine neutrophils. J. Immunol. 144, 2702–2711 (1990).
Husemann, J., Obstfeld, A., Febbraio, M., Kodama, T. & Silverstein, S.C. CD11b/CD18 mediates production of reactive oxygen species by mouse and human macrophages adherent to matrixes containing oxidized LDL. Arterioscler. Thromb. Vasc. Biol. 21, 1301–1305 (2001).
Zarbock, A., Singbartl, K. & Ley, K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J. Clin. Invest. 116, 3211–3219 (2006).
Doerschuk, C.M. Mechanisms of leukocyte sequestration in inflamed lungs. Microcirculation 8, 71–88 (2001).
Eppiheimer, M.J., Wolitzky, B., Anderson, D.C., Labow, M.A. & Granger, D.N. Heterogeneity of expression of E- and P-selectins in vivo. Circ. Res. 79, 560–569 (1996).
Lutz, H.U. et al. Naturally occurring anti–band-3 antibodies and complement together mediate phagocytosis of oxidatively stressed human erythrocytes. Proc. Natl. Acad. Sci. USA 84, 7368–7372 (1987).
Wang, R.H. & Phillips, G., Jr, Medof, M.E. & Mold, C. Activation of the alternative complement pathway by exposure of phosphatidylethanolamine and phosphatidylserine on erythrocytes from sickle cell disease patients. J. Clin. Invest. 92, 1326–1335 (1993).
Zennadi, R. et al. Role and regulation of sickle red cell interactions with other cells: ICAM-4 and other adhesion receptors. Transfus. Clin. Biol. 15, 23–28 (2008).
Gaarder, A., Jonsen, J., Laland, S., Hellem, A. & Owren, P.A. Adenosine diphosphate in red cells as a factor in the adhesiveness of human blood platelets. Nature 192, 531–532 (1961).
Santos, M.T. et al. Enhancement of platelet reactivity and modulation of eicosanoid production by intact erythrocytes. A new approach to platelet activation and recruitment. J. Clin. Invest. 87, 571–580 (1991).
Coller, B.S. Leukocytosis and ischemic vascular disease morbidity and mortality: is it time to intervene? Arterioscler. Thromb. Vasc. Biol. 25, 658–670 (2005).
Goel, M.S. & Diamond, S.L. Adhesion of normal erythrocytes at depressed venous shear rates to activated neutrophils, activated platelets, and fibrin polymerized from plasma. Blood 100, 3797–3803 (2002).
Wakefield, T.W., Myers, D.D. & Henke, P.K. Mechanisms of venous thrombosis and resolution. Arterioscler. Thromb. Vasc. Biol. 28, 387–391 (2008).
Dagia, N.M. et al. G-CSF induces E-selectin ligand expression on human myeloid cells. Nat. Med. 12, 1185–1190 (2006).
Arimura, K. et al. Acute lung injury in a healthy donor during mobilization of peripheral blood stem cells using granulocyte-colony stimulating factor alone. Haematologica 90, ECR10 (2005).
Paszty, C. et al. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science 278, 876–878 (1997).
Acknowledgements
We thank C. Jakubzick for help establishing the lung injury model and B. Wolitzky (Immune Tolerance Network) for the 9A9 antibody to E-selectin. This work was supported by US National Institutes of Health grants R01 HL69438 to P.S.F. and T32 HL07824 to J.C. and by a Scientist Development Grant from the American Heart Association to A.H. (0735165N). P.S.F. is supported by an Established Investigator Award from the American Heart Association. A.H. is the recipient of a Ramón y Cajal fellowship from the Spanish Ministry of Science and Innovation.
Author information
Authors and Affiliations
Contributions
A.H. conceived the study, performed experiments, analyzed data and wrote the manuscript; J.C. performed experiments and analyzed data; A.J.P. maintained and generated the mice used in this study; J.-E.J. performed experiments and analyzed data; E.Y.C. performed experiments and analyzed data; and P.S.F. conceived the study, supervised the overall project, analyzed data and wrote the manuscript.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–11, Supplementary Tables 1 and 2 and Supplementary Methods (PDF 543 kb)
Supplementary Video 1
nRBC captures are mediated by the leading edge of adherent PMNs. Adherent leukocytes in venules of C57BL/6 mice treated with TNF-α were imaged following the intravenous injection of PE-conjugated anti-L-selectin (red, 0.5 μg) and FITC-conjugated anti-LFA-1 (clone M17/4; green, 1 μg). L-selectin clusters identify the trailing edge of adherent leukocytes. Brightfield images of nRBC interactions with PMNs in inflamed venules were captured 180 min after cytokine administration. (MOV 1362 kb)
Supplementary Video 2
Platelets interact mostly with leukocyte microdomains at the leading edge. Platelets were labeled by anti-CD41 (red, 1 μg / mouse) and the trailing edge with anti-L-selectin (blue, 0.02 mg/Kg) in a TNF-α-stimulated mouse. Real time is shown in the left upper corner (h:min:s). (MOV 1643 kb)
Supplementary Video 3
Regional activation of αMβ2 integrin at the leading edge of adherent leukocytes mediates fluosphere capture. TNF-α-treated C57BL/6 mice were injected with PE-conjugated anti-L-selectin (red; 0.5 μg) to label the trailing edge of adherent leukocytes. Fluosphere interactions with adherent leukocytes in inflamed venules were imaged 180 min after cytokine treatment, immediately upon the injection of albumin-coated fluospheres (green) through a catheter placed in the left carotid artery. (MOV 7932 kb)
Supplementary Video 4a
Platelet-WBC interactions are markedly induced by anti-H2d administration in Balb/c mice. Platelets were labeled by anti-CD41 (red, 1 μg / mouse) and the trailing edge with anti-L-selectin (blue, 0.02 mg/Kg). (4a) Sequence of images just before anti-H2d administration. (MOV 4785 kb)
Supplementary Video 4b
Platelet-WBC interactions are markedly induced by anti-H2d administration in Balb/c mice. Platelets were labeled by anti-CD41 (red, 1 μg / mouse) and the trailing edge with anti-L-selectin (blue, 0.02 mg/Kg). (4b) Sequence of images taken after anti-H2d injection. Note the increase in platelet captures by leukocytes and also the interactions of non-labeled RBCs with adherent leukocytes. (MOV 4665 kb)
Rights and permissions
About this article
Cite this article
Hidalgo, A., Chang, J., Jang, JE. et al. Heterotypic interactions enabled by polarized neutrophil microdomains mediate thromboinflammatory injury. Nat Med 15, 384–391 (2009). https://doi.org/10.1038/nm.1939
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.1939
This article is cited by
-
Behavioural immune landscapes of inflammation
Nature (2022)
-
Vaso-occlusive crisis in sickle cell disease: a vicious cycle of secondary events
Journal of Translational Medicine (2021)
-
Heme induces significant neutrophil adhesion in vitro via an NFκB and reactive oxygen species-dependent pathway
Molecular and Cellular Biochemistry (2021)
-
Venous thromboembolism and COVID-19: a single center experience from an academic tertiary referral hospital of Northern Italy
Internal and Emergency Medicine (2021)
-
CXCR4hi effector neutrophils in sickle cell anemia: potential role for elevated circulating serotonin (5-HT) in CXCR4hi neutrophil polarization
Scientific Reports (2020)