Abstract
Variola major (smallpox) infection claimed hundreds of millions lives before it was eradicated by a simple vaccination strategy: epicutaneous application of the related orthopoxvirus vaccinia virus (VACV) to superficially injured skin (skin scarification, s.s.)1. However, the remarkable success of this strategy was attributed to the immunogenicity of VACV rather than to the unique mode of vaccine delivery. We now show that VACV immunization via s.s., but not conventional injection routes, is essential for the generation of superior T cell–mediated immune responses that provide complete protection against subsequent challenges, independent of neutralizing antibodies. Skin-resident effector memory T cells (TEM cells) provide complete protection against cutaneous challenge, whereas protection against lethal respiratory challenge requires both respiratory mucosal TEM cells and central memory T cells (TCM cells). Vaccination with recombinant VACV (rVACV) expressing a tumor antigen was protective against tumor challenge only if delivered via the s.s. route; it was ineffective if delivered by hypodermic injection. The clinically safer nonreplicative modified vaccinia Ankara virus (MVA) also generated far superior protective immunity when delivered via the s.s. route compared to intramuscular (i.m.) injection as used in MVA clinical trials. Thus, delivery of rVACV-based vaccines, including MVA vaccines, through physically disrupted epidermis has clear-cut advantages over conventional vaccination via hypodermic injection.
Similar content being viewed by others
Main
Smallpox was eradicated worldwide by immunization with VACV delivered to skin with a bifurcated needle (s.s.)1,2,3. We tested the hypothesis that rVACV immunization via s.s. is crucial for the generation of superior protective immune responses. Using an established mouse model of VACV delivered by the s.s. route4,5, we immunized C57BL/6 mice with rVACV by either s.s. or hypodermic injection routes. We found that mice vaccinated by s.s. with rVACV generated more interferon-γ (IFN-γ)-producing CD8+ T cells (Fig. 1a), a superior recall IFN-γ response (Fig. 1b) and superior humoral responses (Fig. 1c,d). We next immunized mice with rVACV by subcutaneous (s.c.) injection, with or without simultaneous epidermal disruption with a scarification needle (mock s.s.). Cellular and humoral responses were equivalent in these two groups and were inferior to the rVACV-s.s. group (Supplementary Fig. 1a,b). Heat-inactivated rVACV delivered via s.s. also failed to induce a substantial cellular or antibody response (Supplementary Fig. 1c,d). These data suggest that live VACV infection of disrupted epidermis is essential for the superiority of s.s over other vaccination routes. We compared viral messenger RNA and protein expression after delivery of rVACV via s.s. and s.c. routes. Despite equivalent initial viral loads (Supplementary Fig. 2a), s.s. delivery resulted in markedly higher viral gene expression at the inoculation site (Supplementary Fig. 2b,c). Epidermal and follicular keratinocytes were infected uniformly after s.s., but not s.c., immunization (Supplementary Fig. 2c), resulting in a higher available antigen dose.
(a) Quantification of the frequencies of IFN-γ+CD8+ T cells in spleens 7 d after immunization by intracellular IFN-γ staining. UI, unimmunized control. (b) Quantification of IFN-γ production from memory splenocytes at 5 weeks after immunization by in vitro re-stimulation assay. (c) Serum VACV-specific IgG determined by ELISA at the indicated time points after immunization. Each data point represents the average absorbance at 450 nm ± s.e.m. n = 5 in each group. (d) Serum VACV-specific IgG levels determined 11 weeks after immunization. n = 8–10 in each group. UD, undetectable; reciprocal titers are shown. Data are representative results from two (a,c,d) or three (b) independent experiments.
We recently showed that, after rVACV scarification, antigen-specific T cells are imprinted with skin-homing markers in draining lymph nodes (DLNs) and rapidly migrate into infected skin4. We predicted that rVACV delivery via s.s. route would be highly protective against cutaneous challenge. We challenged mice previously immunized with rVACV (rVACV memory mice) via various routes (s.s., s.c., intradermal (i.d.), i.m. and intraperitoneal (i.p.)) with a secondary rVACV infection to skin and measured viral load at the challenge site 6 d later. Mice immunized via s.s. had completely cleared virus from the infected site (a more than six-log viral load reduction), whereas mice immunized by all other routes showed incomplete viral clearance (Fig. 2a). These data indicate that s.s. rVACV vaccination induces dramatically superior protective immunity in skin.
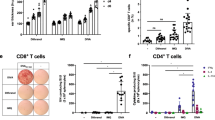
(a) Skin viral load measured by real-time PCR on day 6 after cutaneous challenge of WT mice immunized with VACV 6 weeks previously. (b) Skin viral load on day 6 after cutaneous challenge of μMT or WT mice immunized with VACV 6 weeks before challenge. In some WT immune mice, T cells were depleted before and during the challenge. Data are pooled results from three independent experiments. Error bars represent means ± s.e.m. n = 9–15 per group per experiment. (c,d) The frequencies of OT-I T cells in the CD45+ leukocyte population in skin and blood either before (c) or after (d) challenge. FACS plots were gated on CD45+ leukocyte populations in skin. Numbers in the plots represent the frequencies of OT-I T cells in the CD45+ cell population. (e) Frequency of T cells in the blood and the absolute number of T cells in DLN measured immediately before secondary challenge to assess the efficiency of FTY720 blockage of T cell egress from lymphoid tissues. (f) Frequencies of OT-I cells in total viable cell populations in skin tissue and DLN at d 4 after challenge. (g) Cutaneous viral load at day 6 after secondary challenge, as determined by real-time PCR. Data are pooled results from two independent experiments. n = 4 or 5 per group per experiment. (h) Immunohistochemistry staining showing the presence of CD3+ T cells (arrowhead) in skin tissue of s.s.- or i.p.- immunized mice before or after secondary cutaneous rVACV challenge. Scale bar, 5 μm. Photographs shown are representative of nine slides from three mice per group.
The mode of protection against cutaneous challenge was distinct for i.p. and s.s. immunization. The moderate protection after i.p. vaccination could be abrogated by T cell depletion and was entirely absent in μMT mice, which lack the heavy (μ) chain of IgM and do not produce antibodies (Fig. 2b). However, the s.s.-induced protection against skin challenge remained intact in μMT mice and was significantly compromised only after T cell depletion (Fig. 2b). This is consistent with a significantly stronger recall T cell response after cutaneous challenge in s.s.-immunized mice compared to i.p.-immunized mice, in both wild-type (WT) and μMT mice (Supplementary Fig. 3). Therefore, T cell memory generated by rVACV s.s. immunization is both necessary and sufficient for the protection against cutaneous challenge.
We adoptively transferred OT-I–transgenic T cells, specific for the ovalbumin peptide SIINFEKL (Ova257–264), 1 d before immunization with an rVACV that expresses Ova257–264 under the control of an early gene promoter6. OT-I cells were readily found in the skin of s.s.-immunized memory mice, both before (Fig. 2c) and 4 d after (Fig. 2d) secondary cutaneous challenge, indicating efficient generation and recruitment of skin-homing TEM cells by the s.s. route. We have reported previously that both VACV-specific TEM cells and VACV-specific TCM cells are generated after s.s. with rVACV4. We next asked whether the protective skin immunity in s.s.-immunized mice is mediated by TEM cells already resident to skin before secondary challenge, or by newly activated TCM cells from the lymph nodes. T cell egress from lymph nodes was blocked by treating mice before and during cutaneous challenge with FTY720, a sphingosine-1-phosphate antagonist that blocks egress of lymphocytes from secondary lymphoid organs7,8. FTY720 treatment induced pronounced lymphocytopenia via sequestration of lymphocytes in the lymph nodes (Fig. 2e)7,8. Moreover, it led to a decrease in the number and frequency of OT-I cells in skin and a concurrent accumulation of OT-I cells in DLN in cutaneously challenged mice (Fig. 2f). Despite this, viral clearance from skin was unaffected by FTY720 treatment (Fig. 2g). These results suggest that the skin-resident TEM cells generated by the original rVACV s.s. are highly effective in the control of virus upon subsequent cutaneous challenge. Activation of OT-I TCM cells in DLN and their subsequent recruitment to skin after challenge was not required for the complete and rapid elimination of virus by 6 d (Fig. 2e–g). Because FTY720 treatment blocked T cell egress from the lymph nodes (Fig. 2e), the TCM cells were unable to migrate from the lymph nodes to skin and participate in the virus clearance. Therefore, the observed viral clearance (Fig. 2g) was independent of TCM cells. This role of tissue-resident TEM cells is supported by recent studies of herpes simplex virus infection9,10. The efficient generation of the skin-resident TEM cell population was achieved only by rVACV immunization via the s.s. route, as we found increased numbers of CD3+ T cells in skin tissues of s.s.-immunized mice, but not mice in the injection groups, by histopathological analysis both before and after secondary cutaneous challenge (Fig. 2h and Supplementary Fig. 4). These observations explain the superior protection against cutaneous challenge afforded by rVACV s.s. immunization.
These data prompted us to evaluate the efficacy of rVACV scarification for protection against a tumor challenge in skin. We challenged mice previously immunized with rVACV expressing OVA257–264 via s.s., i.d., s.c., i.m. or i.p. routes with the OVA-expressing B16 melanoma cell line MO5 delivered i.d. (ref. 11). One week after MO5 implantation, tumor growth was evident at the injection site of unimmunized control mice (Fig. 3a), all of which experienced rapid tumor growth and became moribund within 1 month (Fig. 3b). By 5 weeks after challenge, s.c.-immunized mice experienced 100% mortality, i.d.- and i.m.-immunized mice showed 75% mortality and i.p.-immunized mice experienced 50% mortality (Fig. 3b), with all surviving mice harboring large tumors (Fig. 3a). In contrast, s.s.-immunized mice showed 100% survival by the end of the experiment (44 d after challenge) (Fig. 3b). The tumors that did develop in s.s.-immunized mice were considerably smaller than in all other mice studied and grew much more slowly or not at all (Fig. 3a), suggesting an ongoing immune response. These results indicate that rVACV immunization via s.s. is highly effective in generating skin-targeted memory T cells recognizing antigens on cutaneous tumors.
(a) Tumor volumes measured at the indicated time points after MO5 melanoma challenge of mice immunized with rVACV via various routes. Each data point represents the mean tumor volume in that group. Error bars represent means ± s.e.m. (b) Survival rate of mice after MO5 implantation. n = 8 mice per group. The data shown are the pooled results of two independent experiments.
Smallpox, caused by variola major virus, is transmitted primarily via respiratory droplets. To investigate whether rVACV scarification also provides superior protection in respiratory tissues, we intranasally challenged rVACV memory mice previously immunized by s.s., i.d., s.c., i.m. or i.p. routes with lethal doses of pathogenic Western Reserve (WR)-VACV. Mice immunized via the s.c., i.d. or i.m. routes all showed marked weight loss after challenge and were only partially protected from lethality (Fig. 4a,b). In contrast, mice immunized by the s.s. route not only survived but were completely protected from clinical illness (as judged by absence of weight loss) (Fig. 4a,b). Vaccination via s.s. protected mice against the lethal respiratory challenge even at three-log lower doses of rVACV vaccine (Supplementary Fig. 5).
(a,b) Change of body weight (BW) (a) and survival rate (b) of rVACV-immune WT mice after lethal intranasal WR-VACV challenge. (c,d) Change of BW (c) and survival rate (d) of rVACV s.s.–immunized and WR-VACV–challenged WT or μMT mice, with or without T cell depletion at the time of challenge. (e,f) Change of BW (e) and survival rate (f) of rVACV s.s.–immune μMT mice after WR-VACV challenge, with or without FTY720 treatment. (g,h) Change of BW (g) and survival rate (h) of MVA-immune WT mice after intranasal WR-VACV challenge. Immune mice were challenged with WR-VACV intranasally at 6 weeks (a–d, g,h) or 16 weeks (e,f) after immunization. Unimmunized controls were included in all experiments. Data are representative results from two or three independent experiments (n = 10 per group).
We used μMT immune mice or WT immune mice depleted of T cells to explore the mechanism of this protection against lethal intranasal challenge. After rVACV s.s. immunization, both μMT immune mice and T cell–depleted WT immune mice were fully protected from illness and death, indicating that either T cell memory or antibodies were sufficient to provide complete protection against this lethal challenge (Fig. 4c,d). Only T cell depletion in s.s.-immunized μMT memory mice abrogated protection (Fig. 4c,d). FTY720-treated, s.s.-immunized μMT memory mice were partially protected against the lethality of the intranasal challenge (Fig. 4e,f and Supplementary Fig. 6). This suggests that protective TEM cells lining upper respiratory mucosa are generated by s.s. immunization. The two different clinical outcomes after intranasal challenge of μMT memory mice with and without FTY720 treatment suggests that activation of TCM cells in the lymph nodes draining respiratory mucosa, and the subsequent recruitment of TEM cells to the respiratory tract, are essential for full protection against respiratory infectious challenge. Collectively, these data indicate that even in the complete absence of an antibody response, VACV-specific TCM and TEM cells together provide complete protective immunity against lethal respiratory challenge in s.s.-immunized mice.
Although smallpox has been eradicated in the wild and exists only in frozen stocks in government facilities, concerns about bioterrorism have raised questions about the need for reintroduction of widespread smallpox vaccination with VACV. However, in the absence of an established smallpox epidemic, mass vaccination of the general public with VACV is precluded by an unacceptably high incidence of morbidity after vaccination, particularly in individuals with atopic disorders12,13,14,15,16,17,18. The nonreplicating MVA is an attractive alternative to VACV, as it has an impressive safety profile19,20,21,22. MVA vaccines are typically delivered by i.m. injection. Skin scarification has not been tested in MVA vaccination studies. We immunized C57BL/6 mice with MVA via s.s. This procedure elicited cellular and humoral immune responses to VACV in a dose-dependent manner and provided dose-dependent protection against lethal intranasal challenge with WR-VACV (Supplementary Fig. 7). Notably, a single immunization with 2 × 106 plaque-forming units (PFU) of MVA via the s.s. route, but not i.m. injection, provided complete protection against morbidity and mortality in this model (Fig. 4g,h). This result is consistent with the markedly stronger immune responses after MVA s.s. immunization (Supplementary Fig. 8).
Taken together, the data presented here establish that immunization with rVACV vaccines, including nonreplicative strains, via superficially injured skin (that is, s.s.), provides robust and anatomically flexible protection associated with a highly effective peripheral TEM and TCM cell response without requirement for neutralizing antibodies. The mechanism by which s.s. is so much more effective than other immunization routes is incompletely understood. VACV infection of epidermal keratinocytes after s.s. may trigger a cascade of proinflammatory molecule production23,24,25, as well as provide a higher dose of antigen, both of which may enhance the generation of optimal immunity. We hope that these insights will pave the way for the design of safe affordable and effective VACV-based immunization for infectious diseases and cancer.
Methods
Mice, viruses and infection.
Our experiments using mice were in compliance with the guidelines set out by the Center for Animal Resources and Comparative Medicine at Harvard Medical School. C57BL/6 and μMT mice were from Jackson Laboratory. We bred Thy1.1+ OT-I Rag1−/− mice in a Harvard Medical School animal facility. We expanded and titered rVACV expressing both EGFP and the OT-I T cell epitope OVA257–264 (ref. 6) and WR-VACV stocks by standard procedures26. We immunized mice with 2 × 106 PFU VACV or MVA (ACAM3000MVA) via the s.s., i.d., s.c., i.m., or i.p. routes. We performed s.s. as previously described4. Six weeks after immunization, we challenged mice either cutaneously with rVACV (2 × 106 PFU) or intranasally with WR-VACV (2 × 106 PFU). For histology and FTY720 treatment studies, we challenged mice at 15 and 16 weeks after immunization, respectively. We killed mice that had lost over 25% of their original body weight.
Intracellular cytokine staining.
To prepare target cells, we infected naive C57BL/6 splenocytes with rVACV at a multiplicity of infection of 5 for 8 h followed by three washes. We incubated splenocytes from immunized and control mice with target cells (1:1 ratio) in a 96-well plate for 6 h in the presence of GolgiStop (BD Pharmingen) and subsequently performed surface and intracellular staining with the Cytoperm/Cytofix kit (BD Pharmingen) according to the manufacturer's protocol. We used antibodies (BD Pharmingen) to mouse CD16/CD32 (2.4G2), CD3e (conjugated to FITC) (145-2C11), CD8α (conjugated to PerCP) (53-6.7), IFN-γ (conjugated to phycoerythrin) (XMG1.2) and IFN-γ isotype control (X40, catalog number 340760) for XMG1.2. We analyzed samples on a FACSCanto flow cytometer (Becton Dickinson). We analyzed data with FlowJo software (Tree Star).
In vitro re-stimulation assay.
We cultured target cells (prepared as described above) with splenocytes or inguinal (draining) lymph node cells from immune or control mice (1:1 ratio) in 96-well plates for 48 h. We measured IFN-γ concentration in the supernatant by ELISA using monoclonal antibody pairs to IFN-γ (BD Pharmingen), according to the manufacturer's instructions.
Enzyme-linked immunosorbent assay for serum vaccinia-specific antibody.
We coated ELISA plates with rVACV-infected HeLa cell lysates at 4 °C overnight, blocked the plates with PBS containing 10% FCS and 0.1% Tween-20 for 2 h and then added serum sample dilutions and incubated the plates at 4 °C for 1 h. We detected antibody with horseradish peroxidase–conjugated goat antibody to mouse IgG (Southern Biotech) and tetramethylbenzidine substrate reagent (BD Pharmingen). We determined A450 with a Vmax Spectrophotometer (Molecular Devices) and calculated end-point titers as the highest dilution with an absorbance value >0.10 after subtraction of background wells assayed without antigen.
Determination of cutaneous viral load.
We determined skin viral load by real-time PCR, as previously described4.
In vivo depletion of T cells.
We injected mice i.p. with antibody to mouse CD8 (clone 2.43, 0.5 mg) and mouse CD4 (clone GK1.5, 0.5 mg) (Bio X Cell) 2 d before and on days 0, 4 and 8 after secondary challenge. This regimen depleted >99% of CD8+ and CD4+ T cells (data not shown).
Adoptive transfer and detection of OT-I cells in skin.
We intravenously injected 2 × 105 OT-I cells from Thy1.1+ OT-I Rag1−/− mice into Thy1.2+ C57BL/6 mice 1 d before rVACV s.s. on ear skin (2 × 106 PFU). We challenged mice with 2 × 106 PFU rVACV on the ear 6 weeks after immunization and collected ears and blood samples either before challenge or 4 d after challenge. We separated ears into dorsal and ventral halves and incubated them in HBSS containing 1 mg ml−1 collagenase A and 40 μg ml−1 DNase I (Roche Diagnostics) at 37 °C for 2 h. We then added HBSS containing 2 mM EDTA and 5% FCS to stop the digestion. After filtering the cell suspension through 70-μm cell strainers and red cell removal, we stained cells with CD45.2-specific antibody conjugated to phycoerythrin (104) and Thy1.1-specific antibody conjugated to PerCP (OX-7) (BD Pharmingen) for flow cytometry analysis.
FTY720 treatment.
Sixteen weeks after rVACV s.s. immunization, we i.p. injected previously immunized mice daily with 1 mg per kg body weight FTY720 (2-amino-2-(2[4-octylphenyl]ethyl)-1,3-propanediol hydrochloride, provided by V. Brinkmann at Novartis Pharmaceuticals) or PBS (mock control). After one or two doses, we challenged the mice cutaneously or intranasally.
Immunohistochemical staining for T cells in skin.
We challenged WT mice immunized with rVACV via the s.s. or i.p. route on tail skin at 15 weeks after immunization. We collected challenged skin tissues either 1 d before or 4 d after challenge. We performed sectioning and immunohistochemical staining of skin samples for CD3+ T cell detection as previously described4. We obtained images with a Nikon E600 microscope and a Nikon EDX-35 digital camera.
Tumor challenge.
Six weeks after rVACV immunization, we i.d. challenged memory and control naive mice with 3 × 105 MO5 cells, an ovalbumin gene–transfected B16 melanoma cell line11. We measured the longest diameter (L) and the perpendicular diameter (W) of the local tumor on days 9, 12, 18, 25, 30, 37 and 44 after challenge and calculated tumor volume with the formula 4πW2L / 3.
Statistical analysis.
We performed statistical comparisons with two-tailed unpaired Student's t test, Mann-Whitney test (for viral load data) and log-rank test (for survival curves) using Prism software. We used area under the curve to compare weight loss between groups. P < 0.05 was considered significant.
Additional methods.
Detailed methodology is described in the Supplementary Methods.
References
Stewart, A.J. & Devlin, P.M. The history of the smallpox vaccine. J. Infect. 52, 329–334 (2006).
Hammarlund, E. et al. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 9, 1131–1137 (2003).
Demkowicz, W.E. Jr., Littaua, R.A., Wang, J. & Ennis, F.A. Human cytotoxic T-cell memory: long-lived responses to vaccinia virus. J. Virol. 70, 2627–2631 (1996).
Liu, L., Fuhlbrigge, R.C., Karibian, K., Tian, T. & Kupper, T.S. Dynamic programming of CD8+ T cell trafficking after live viral immunization. Immunity 25, 511–520 (2006).
Tian, T. et al. Overexpression of IL-1α in skin differentially modulates the immune response to scarification with vaccinia virus. J. Invest. Dermatol. 129, 70–78 (2009).
Norbury, C.C., Malide, D., Gibbs, J.S., Bennink, J.R. & Yewdell, J.W. Visualizing priming of virus-specific CD8+ T cells by infected dendritic cells in vivo. Nat. Immunol. 3, 265–271 (2002).
Matloubian, M. et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427, 355–360 (2004).
Pinschewer, D.D. et al. FTY720 immunosuppression impairs effector T cell peripheral homing without affecting induction, expansion and memory. J. Immunol. 164, 5761–5770 (2000).
Gebhardt, T. et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 10, 524–530 (2009).
Woodland, D.L. & Kohlmeier, J.E. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat. Rev. Immunol. 9, 153–161 (2009).
Falo, L.D. Jr., Kovacsovics-Bankowski, M., Thompson, K. & Rock, K.L. Targeting antigen into the phagocytic pathway in vivo induces protective tumour immunity. Nat. Med. 1, 649–653 (1995).
Neff, J.M. & Lane, J.M. Vaccinia necrosum following smallpox vaccination for chronic herpetic ulcers. J. Am. Med. Assoc. 213, 123–125 (1970).
Neff, J.M. et al. Complications of smallpox vaccination. I. National survey in the United States, 1963. N. Engl. J. Med. 276, 125–132 (1967).
Neff, J.M. et al. Complications of smallpox vaccination United States 1963. II. Results obtained by four statewide surveys. Pediatrics 39, 916–923 (1967).
Bray, M. Pathogenesis and potential antiviral therapy of complications of smallpox vaccination. Antiviral Res. 58, 101–114 (2003).
Bray, M. & Wright, M.E. Progressive vaccinia. Clin. Infect. Dis. 36, 766–774 (2003).
Vora, S. et al. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin. Infect. Dis. 46, 1555–1561 (2008).
Howell, M.D. et al. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity 24, 341–348 (2006).
Kennedy, J.S. & Greenberg, R.N. IMVAMUNE: modified vaccinia Ankara strain as an attenuated smallpox vaccine. Expert Rev. Vaccines 8, 13–24 (2009).
Drexler, I., Staib, C. & Sutter, G. Modified vaccinia virus Ankara as antigen delivery system: how can we best use its potential? Curr. Opin. Biotechnol. 15, 506–512 (2004).
Parrino, J. et al. Safety, immunogenicity and efficacy of modified vaccinia Ankara (MVA) against Dryvax challenge in vaccinia-naive and vaccinia-immune individuals. Vaccine 25, 1513–1525 (2007).
Phelps, A.L., Gates, A.J., Hillier, M., Eastaugh, L. & Ulaeto, D.O. Comparative efficacy of modified vaccinia Ankara (MVA) as a potential replacement smallpox vaccine. Vaccine 25, 34–42 (2007).
Kupper, T.S. & Fuhlbrigge, R.C. Immune surveillance in the skin: mechanisms and clinical consequences. Nat. Rev. Immunol. 4, 211–222 (2004).
Kupper, T.S. & Groves, R.W. The interleukin-1 axis and cutaneous inflammation. J. Invest. Dermatol. 105, 62S–66S (1995).
Partidos, C.D. & Muller, S. Decision-making at the surface of the intact or barrier disrupted skin: potential applications for vaccination or therapy. Cell. Mol. Life Sci. 62, 1418–1424 (2005).
Earl, P.L., Cooper, N., Wyatt, L.S., Moss, B. & Carroll, M.W. Preparation of cell cultures and vaccinia virus stocks. in Current Protocols in Molecular Biology Vol. 2 (eds. Ausubel, F.M. et al.) Ch.16, unit 16.16,14 (John Wiley & Sons, Hoboken, New Jersey, USA, 1998).
Acknowledgements
We thank B. Moss (US National Institute of Health (NIH)) for providing rVACV expressing EGFP and OT-I T cell epitope OVA257–264, as well as WR-VACV. We thank K. Rock (University of Massachusetts Medical School) for providing the MO5 cell line and M. Seaman (Beth Israel Hospital) for providing the MVA stocks. FTY720 was provided by V. Brinkmann at Novartis Pharmaceuticals. This work was supported by NIH–US National Institute of Allergy and Infectious Diseases grants R01 AI042124 and R37 AI025082 to T.S.K. and NIH grant U19AI57330 subcontracted to T.S.K., a Dermatology Foundation Research Career Development Award to L.L. and a Career Development Award and a New Opportunities Grant to L.L. from US National Institute of Allergy and Infectious Diseases–New England Regional Center of Excellence/Biodefense and Emerging Infectious Disease (AI057159).
Author information
Authors and Affiliations
Contributions
L.L. and T.S.K. developed the concept, designed the study, analyzed the data and wrote the paper; L.L. designed the experiments, contributed to the experiments in Figures 1,2,3, analyzed the data and supervised and coordinated all experiments; Q.Z. contributed to the experiments in all of the figures and performed the experiments in Supplementary Figures 3, 4 and 6; T.T. performed experiments in Supplementary Figure 2 and contributed to Figures 2 and 4; K.D. performed experiments in Supplementary Figure 1 and contributed to Figures 2 and 4 and Supplementary Figures 2 and 6; S.K.A. performed experiments in Supplementary Figures 5, 7 and 8 and contributed to Figure 4.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Methods (PDF 1476 kb)
Rights and permissions
About this article
Cite this article
Liu, L., Zhong, Q., Tian, T. et al. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell–mediated immunity. Nat Med 16, 224–227 (2010). https://doi.org/10.1038/nm.2078
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2078
This article is cited by
-
Assessing the generation of tissue resident memory T cells by vaccines
Nature Reviews Immunology (2023)
-
Heat-inactivated modified vaccinia virus Ankara boosts Th1 cellular and humoral immunity as a vaccine adjuvant
npj Vaccines (2022)
-
Epicutaneous immunization with modified vaccinia Ankara viral vectors generates superior T cell immunity against a respiratory viral challenge
npj Vaccines (2021)
-
Local heroes or villains: tissue-resident memory T cells in human health and disease
Cellular & Molecular Immunology (2020)
-
Skin immunisation activates an innate lymphoid cell-monocyte axis regulating CD8+ effector recruitment to mucosal tissues
Nature Communications (2019)