Abstract
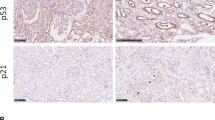
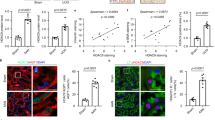
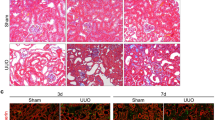
Fibrosis is responsible for chronic progressive kidney failure, which is present in a large number of adults in the developed world. It is increasingly appreciated that acute kidney injury (AKI), resulting in aberrant incomplete repair, is a major contributor to chronic fibrotic kidney disease. The mechanism that triggers the fibrogenic response after injury is not well understood. In ischemic, toxic and obstructive models of AKI, we demonstrate a causal association between epithelial cell cycle G2/M arrest and a fibrotic outcome. G2/M-arrested proximal tubular cells activate c-jun NH2-terminal kinase (JNK) signaling, which acts to upregulate profibrotic cytokine production. Treatment with a JNK inhibitor, or bypassing the G2/M arrest by administration of a p53 inhibitor or the removal of the contralateral kidney, rescues fibrosis in the unilateral ischemic injured kidney. Hence, epithelial cell cycle arrest at G2/M and its subsequent downstream signaling are hitherto unrecognized therapeutic targets for the prevention of fibrosis and interruption of the accelerated progression of kidney disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Humphreys, B.D. et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2, 284–291 (2008).
Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 214, 199–210 (2008).
Coresh, J., Astor, B.C., Greene, T., Eknoyan, G. & Levey, A.S. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am. J. Kidney Dis. 41, 1–12 (2003).
Lameire, N., Jager, K., Van Biesen, W., de Bacquer, D. & Vanholder, R. Chronic kidney disease: a European perspective. Kidney Int. Suppl. S30–S38 (2005).
Forbes, J.M., Hewitson, T.D., Becker, G.J. & Jones, C.L. Ischemic acute renal failure: long-term histology of cell and matrix changes in the rat. Kidney Int. 57, 2375–2385 (2000).
Macedo, E., Bouchard, J. & Mehta, R.L. Renal recovery following acute kidney injury. Curr. Opin. Crit. Care 14, 660–665 (2008).
Kalluri, R. & Neilson, E.G. Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Invest. 112, 1776–1784 (2003).
Liu, Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J. Am. Soc. Nephrol. 15, 1–12 (2004).
Burns, W.C., Kantharidis, P. & Thomas, M.C. The role of tubular epithelial-mesenchymal transition in progressive kidney disease. Cells Tissues Organs 185, 222–231 (2007).
Humphreys, B.D. et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am. J. Pathol. 176, 85–97 (2010).
Nguyen, T.Q. & Goldschmeding, R. Bone morphogenetic protein-7 and connective tissue growth factor: novel targets for treatment of renal fibrosis? Pharm. Res. 25, 2416–2426 (2008).
Qi, W., Chen, X., Poronnik, P. & Pollock, C.A. Transforming growth factor-β/connective tissue growth factor axis in the kidney. Int. J. Biochem. Cell Biol. 40, 9–13 (2008).
Bonventre, J.V. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J. Am. Soc. Nephrol. 14 Suppl 1, S55–S61 (2003).
Price, P.M., Megyesi, J. & Safirstein, R.L. Cell cycle regulation: repair and regeneration in acute renal failure. Kidney Int. 66, 509–514 (2004).
Tanaka, H. et al. Role of the E2F1-p19-p53 pathway in ischemic acute renal failure. Nephron Physiol. 101, 27–34 (2005).
Chkhotua, A.B., Abendroth, D., Froeba, G. & Schelzig, H. Up-regulation of cell cycle regulatory genes after renal ischemia/reperfusion: differential expression of p16(INK4a), p21(WAF1/CIP1) and p27(Kip1) cyclin-dependent kinase inhibitor genes depending on reperfusion time. Transpl. Int. 19, 72–77 (2006).
Melk, A., Schmidt, B.M., Vongwiwatana, A., Rayner, D.C. & Halloran, P.F. Increased expression of senescence-associated cell cycle inhibitor p16INK4a in deteriorating renal transplants and diseased native kidney. Am. J. Transplant. 5, 1375–1382 (2005).
Jiang, M. & Dong, Z. Regulation and pathological role of p53 in cisplatin nephrotoxicity. J. Pharmacol. Exp. Ther. 327, 300–307 (2008).
Price, P.M., Safirstein, R.L. & Megyesi, J. The cell cycle and acute kidney injury. Kidney Int. 76, 604–613 (2009).
Molitoris, B.A. et al. siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J. Am. Soc. Nephrol. 20, 1754–1764 (2009).
Yu, C.C., Woods, A.L. & Levison, D.A. The assessment of cellular proliferation by immunohistochemistry: a review of currently available methods and their applications. Histochem. J. 24, 121–131 (1992).
Crosio, C. et al. Mitotic phosphorylation of histone H3: spatio-temporal regulation by mammalian Aurora kinases. Mol. Cell. Biol. 22, 874–885 (2002).
Silva, F.G., Nadasdy, T. & Laszik, Z. Immunohistochemical and lectin dissection of the human nephron in health and disease. Arch. Pathol. Lab. Med. 117, 1233–1239 (1993).
Ichimura, T., Hung, C.C., Yang, S.A., Stevens, J.L. & Bonventre, J.V. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am. J. Physiol. Renal Physiol. 286, F552–F563 (2004).
Hendzel, M.J. et al. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106, 348–360 (1997).
Abraham, R.T. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15, 2177–2196 (2001).
Goodarzi, A.A., Block, W.D. & Lees-Miller, S.P. The role of ATM and ATR in DNA damage-induced cell cycle control. Prog. Cell Cycle Res. 5, 393–411 (2003).
Bencokova, Z. et al. ATM activation and signaling under hypoxic conditions. Mol. Cell. Biol. 29, 526–537 (2009).
Hickson, I. et al. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 64, 9152–9159 (2004).
Komarov, P.G. et al. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science 285, 1733–1737 (1999).
Vassilev, L.T. et al. Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1. Proc. Natl. Acad. Sci. USA 103, 10660–10665 (2006).
Horwitz, S.B. Mechanism of action of taxol. Trends Pharmacol. Sci. 13, 134–136 (1992).
Ishani, A. et al. Acute kidney injury increases risk of ESRD among elderly. J. Am. Soc. Nephrol. 20, 223–228 (2009).
Leask, A. & Abraham, D.J. TGF-β signaling and the fibrotic response. FASEB J. 18, 816–827 (2004).
Zeisberg, M. et al. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J. Biol. Chem. 282, 23337–23347 (2007).
Qi, W. et al. Integrated actions of transforming growth factor-β1 and connective tissue growth factor in renal fibrosis. Am. J. Physiol. Renal Physiol. 288, F800–F809 (2005).
Okada, H. et al. Connective tissue growth factor expressed in tubular epithelium has a pivotal role in renal fibrogenesis. J. Am. Soc. Nephrol. 16, 133–143 (2005).
Ikawa, Y. et al. Neutralizing monoclonal antibody to human connective tissue growth factor ameliorates transforming growth factor-β–induced mouse fibrosis. J. Cell. Physiol. 216, 680–687 (2008).
Shi-Wen, X., Leask, A. & Abraham, D. Regulation and function of connective tissue growth factor/CCN2 in tissue repair, scarring and fibrosis. Cytokine Growth Factor Rev. 19, 133–144 (2008).
Stark, G.R. & Taylor, W.R. Control of the G2/M transition. Mol. Biotechnol. 32, 227–248 (2006).
Taylor, W.R. & Stark, G.R. Regulation of the G2/M transition by p53. Oncogene 20, 1803–1815 (2001).
Bunz, F. et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282, 1497–1501 (1998).
Kailong, L. et al. P53-Rb signaling pathway is involved in tubular cell senescence in renal ischemia/reperfusion injury. Biocell 31, 213–223 (2007).
Megyesi, J., Price, P.M., Tamayo, E. & Safirstein, R.L. The lack of a functional p21(WAF1/CIP1) gene ameliorates progression to chronic renal failure. Proc. Natl. Acad. Sci. USA 96, 10830–10835 (1999).
Zahedi, K. et al. Stathmin-deficient mice develop fibrosis and show delayed recovery from ischemic-reperfusion injury. Am. J. Physiol. Renal Physiol. 290, F1559–F1567 (2006).
Hong, H. et al. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature 460, 1132–1135 (2009).
Kawamura, T. et al. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature 460, 1140–1144 (2009).
Marion, R.M. et al. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature 460, 1149–1153 (2009).
Ma, F.Y., Sachchithananthan, M., Flanc, R.S. & Nikolic-Paterson, D.J. Mitogen activated protein kinases in renal fibrosis. Front. Biosci. (Schol. Ed.) 1, 171–187 (2009).
Park, K.M. et al. Inducible nitric oxide synthase is an important contributor to prolonged protective effects of ischemic preconditioning in the mouse kidney. J. Biol. Chem. 278, 27256–27266 (2003).
Hung, C.C., Ichimura, T., Stevens, J.L. & Bonventre, J.V. Protection of renal epithelial cells against oxidative injury by endoplasmic reticulum stress preconditioning is mediated by ERK1/2 activation. J. Biol. Chem. 278, 29317–29326 (2003).
Ichimura, T. et al. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J. Clin. Invest. 118, 1657–1668 (2008).
Acknowledgements
This work was supported by US National Institutes of Health (NIH) grants DK39773 and DK72381 to J.V.B. and DK074030 to J.V.S. L.Y. was supported by a fellowship from the International Society of Nephrology.
Author information
Authors and Affiliations
Contributions
L.Y. and J.V.B. designed the experiments and wrote the manuscript. L.Y. performed experiments and collected and analyzed data. J.V.B. supervised the project. J.V.S. designed the in vitro rescue experiment and advised on cell biology. T.Y.B. helped collect data and edited the manuscript. C.R.B. helped with making lentivirus shRNA specific for ATM. All authors discussed the results and implications and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 (PDF 2685 kb)
Rights and permissions
About this article
Cite this article
Yang, L., Besschetnova, T., Brooks, C. et al. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med 16, 535–543 (2010). https://doi.org/10.1038/nm.2144
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2144
This article is cited by
-
Mechanisms of norcantharidin against renal tubulointerstitial fibrosis
Pharmacological Reports (2024)
-
The NephroCheck bedside system for detecting stage 3 acute kidney injury after open thoracoabdominal aortic repair
Scientific Reports (2023)
-
14-3-3ζ inhibits maladaptive repair in renal tubules by regulating YAP and reduces renal interstitial fibrosis
Acta Pharmacologica Sinica (2023)
-
HCK induces macrophage activation to promote renal inflammation and fibrosis via suppression of autophagy
Nature Communications (2023)
-
HDAC9-mediated epithelial cell cycle arrest in G2/M contributes to kidney fibrosis in male mice
Nature Communications (2023)