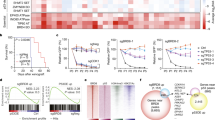
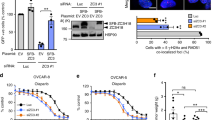
Abstract
BRCA1, a well-known tumor suppressor with multiple interacting partners, is predicted to have diverse biological functions. However, so far its only well-established role is in the repair of damaged DNA and cell cycle regulation. In this regard, the etiopathological study of low-penetrant variants of BRCA1 provides an opportunity to uncover its other physiologically important functions. Using this rationale, we studied the R1699Q variant of BRCA1, a potentially moderate-risk variant, and found that it does not impair DNA damage repair but abrogates the repression of microRNA-155 (miR-155), a bona fide oncomir. Mechanistically, we found that BRCA1 epigenetically represses miR-155 expression via its association with HDAC2, which deacetylates histones H2A and H3 on the miR-155 promoter. We show that overexpression of miR-155 accelerates but the knockdown of miR-155 attenuates the growth of tumor cell lines in vivo. Our findings demonstrate a new mode of tumor suppression by BRCA1 and suggest that miR-155 is a potential therapeutic target for BRCA1-deficient tumors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
07 November 2011
In the version of this article initially published, Shyam K Sharan's affiliation was erroneously listed as affiliation 7, when it should have been affiliation 1. The error has been corrected for the PDF and HTML versions of this article.
References
O'Donovan, P.J. & Livingston, D.M. BRCA1 and BRCA2: breast/ovarian cancer susceptibility gene products and participants in DNA double-strand break repair. Carcinogenesis 31, 961–967 (2010).
Venkitaraman, A.R. Linking the cellular functions of BRCA genes to cancer pathogenesis and treatment. Annu. Rev. Pathol. 4, 461–487 (2009).
Deng, C.X. BRCA1: cell cycle checkpoint, genetic instability, DNA damage response and cancer evolution. Nucleic Acids Res. 34, 1416–1426 (2006).
Xia, Y., Pao, G.M., Chen, H.W., Verma, I.M. & Hunter, T. Enhancement of BRCA1 E3 ubiquitin ligase activity through direct interaction with the BARD1 protein. J. Biol. Chem. 278, 5255–5263 (2003).
Baer, R. & Ludwig, T. The BRCA1/BARD1 heterodimer, a tumor suppressor complex with ubiquitin E3 ligase activity. Curr. Opin. Genet. Dev. 12, 86–91 (2002).
Bork, P. et al. A superfamily of conserved domains in DNA damage-responsive cell cycle checkpoint proteins. FASEB J. 11, 68–76 (1997).
Abbott, D.W. et al. BRCA1 expression restores radiation resistance in BRCA1-defective cancer cells through enhancement of transcription-coupled DNA repair. J. Biol. Chem. 274, 18808–18812 (1999).
Li, S. et al. Binding of CtIP to the BRCT repeats of BRCA1 involved in the transcription regulation of p21 is disrupted upon DNA damage. J. Biol. Chem. 274, 11334–11338 (1999).
Cantor, S.B. et al. BACH1, a novel helicase-like protein, interacts directly with BRCA1 and contributes to its DNA repair function. Cell 105, 149–160 (2001).
Yu, X., Chini, C.C., He, M., Mer, G. & Chen, J. The BRCT domain is a phospho-protein binding domain. Science 302, 639–642 (2003).
Cortez, D., Wang, Y., Qin, J. & Elledge, S.J. Requirement of ATM-dependent phosphorylation of brca1 in the DNA damage response to double-strand breaks. Science 286, 1162–1166 (1999).
Ruffner, H., Jiang, W., Craig, A.G., Hunter, T. & Verma, I.M. BRCA1 is phosphorylated at serine 1497 in vivo at a cyclin-dependent kinase 2 phosphorylation site. Mol. Cell Biol. 19, 4843–4854 (1999).
Chen, J. Ataxia telangiectasia-related protein is involved in the phosphorylation of BRCA1 following deoxyribonucleic acid damage. Cancer Res. 60, 5037–5039 (2000).
Ouchi, M. et al. BRCA1 phosphorylation by Aurora-A in the regulation of G2 to M transition. J. Biol. Chem. 279, 19643–19648 (2004).
Szabo, C.I. & King, M.C. Inherited breast and ovarian cancer. Hum. Mol. Genet. 4 Spec No, 1811–1817 (1995).
Williams, R.S., Lee, M.S., Hau, D.D. & Glover, J.N. Structural basis of phosphopeptide recognition by the BRCT domain of BRCA1. Nat. Struct. Mol. Biol. 11, 519–525 (2004).
Clapperton, J.A. et al. Structure and mechanism of BRCA1 BRCT domain recognition of phosphorylated BACH1 with implications for cancer. Nat. Struct. Mol. Biol. 11, 512–518 (2004).
Lovelock, P.K. et al. Identification of BRCA1 missense substitutions that confer partial functional activity: potential moderate risk variants? Breast Cancer Res. 9, R82 (2007).
Gómez García, E.B. et al. A method to assess the clinical significance of unclassified variants in the BRCA1 and BRCA2 genes based on cancer family history. Breast Cancer Res. 11, R8 (2009).
Chang, S., Biswas, K., Martin, B.K., Stauffer, S. & Sharan, S.K. Expression of human BRCA1 variants in mouse ES cells allows functional analysis of BRCA1 mutations. J. Clin. Invest. 119, 3160–3171 (2009).
Tam, W., Hughes, S.H., Hayward, W.S. & Besmer, P. Avian bic, a gene isolated from a common retroviral site in avian leukosis virus-induced lymphomas that encodes a noncoding RNA, cooperates with c-myc in lymphomagenesis and erythroleukemogenesis. J. Virol. 76, 4275–4286 (2002).
van den Berg, A. et al. High expression of B-cell receptor inducible gene BIC in all subtypes of Hodgkin lymphoma. Genes Chromosom. Cancer 37, 20–28 (2003).
Metzler, M., Wilda, M., Busch, K., Viehmann, S. & Borkhardt, A. High expression of precursor microRNA-155/BIC RNA in children with Burkitt lymphoma. Genes Chromosom. Cancer 39, 167–169 (2004).
Kluiver, J. et al. BIC and miR-155 are highly expressed in Hodgkin, primary mediastinal and diffuse large B cell lymphomas. J. Pathol. 207, 243–249 (2005).
O'Connell, R.M. et al. Sustained expression of microRNA-155 in hematopoietic stem cells causes a myeloproliferative disorder. J. Exp. Med. 205, 585–594 (2008).
Costinean, S. et al. Pre-B cell proliferation and lymphoblastic leukemia/high-grade lymphoma in E(mu)-miR155 transgenic mice. Proc. Natl. Acad. Sci. USA 103, 7024–7029 (2006).
Yamamoto, M. et al. miR-155, a Modulator of FOXO3a protein expression, is underexpressed and cannot be upregulated by stimulation of HOZOT, a line of multifunctional Treg. PLoS ONE 6, e16841 (2011).
Kong, W. et al. MicroRNA-155 regulates cell survival, growth, and chemosensitivity by targeting FOXO3a in breast cancer. J. Biol. Chem. 285, 17869–17879 (2010).
Gironella, M. et al. Tumor protein 53-induced nuclear protein 1 expression is repressed by miR-155, and its restoration inhibits pancreatic tumor development. Proc. Natl. Acad. Sci. USA 104, 16170–16175 (2007).
Jiang, S. et al. MicroRNA-155 functions as an OncomiR in breast cancer by targeting the suppressor of cytokine signaling 1 gene. Cancer Res. 70, 3119–3127 (2010).
Kanellopoulou, C. et al. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev. 19, 489–501 (2005).
Deffenbaugh, A.M., Frank, T.S., Hoffman, M., Cannon-Albright, L. & Neuhausen, S.L. Characterization of common BRCA1 and BRCA2 variants. Genet. Test. 6, 119–121 (2002).
Iorio, M.V. et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 65, 7065–7070 (2005).
Volinia, S. et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl. Acad. Sci. USA 103, 2257–2261 (2006).
Brodie, S.G. et al. Multiple genetic changes are associated with mammary tumorigenesis in Brca1 conditional knockout mice. Oncogene 20, 7514–7523 (2001).
Liu, X. et al. Somatic loss of BRCA1 and p53 in mice induces mammary tumors with features of human BRCA1-mutated basal-like breast cancer. Proc. Natl. Acad. Sci. USA 104, 12111–12116 (2007).
Bartel, D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 (2004).
Cable, P.L. et al. Novel consensus DNA-binding sequence for BRCA1 protein complexes. Mol. Carcinog. 38, 85–96 (2003).
Aiyar, S.E., Cho, H., Lee, J. & Li, R. Concerted transcriptional regulation by BRCA1 and COBRA1 in breast cancer cells. Int. J. Biol. Sci. 3, 486–492 (2007).
Yu, X., Wu, L.C., Bowcock, A.M., Aronheim, A. & Baer, R. The C-terminal (BRCT) domains of BRCA1 interact in vivo with CtIP, a protein implicated in the CtBP pathway of transcriptional repression. J. Biol. Chem. 273, 25388–25392 (1998).
Wang, Q., Zhang, H., Kajino, K. & Greene, M.I. BRCA1 binds c-Myc and inhibits its transcriptional and transforming activity in cells. Oncogene 17, 1939–1948 (1998).
Yarden, R.I. & Brody, L.C. BRCA1 interacts with components of the histone deacetylase complex. Proc. Natl. Acad. Sci. USA 96, 4983–4988 (1999).
Wang, R.H. et al. Interplay among BRCA1, SIRT1, and Survivin during BRCA1-associated tumorigenesis. Mol. Cell 32, 11–20 (2008).
Lee, M.S. et al. Comprehensive analysis of missense variations in the BRCT domain of BRCA1 by structural and functional assays. Cancer Res. 70, 4880–4890 (2010).
Rowling, P.J., Cook, R. & Itzhaki, L.S. Toward classification of BRCA1 missense variants using a biophysical approach. J. Biol. Chem. 285, 20080–20087 (2010).
Shang, Y., Hu, X., DiRenzo, J., Lazar, M.A. & Brown, M. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103, 843–852 (2000).
Acknowledgements
We thank J. Acharya, K. Biswas, R. Chittela, I. Daar, K. Reilly and A. Spurdle for helpful discussions and critical review of the manuscript. We also thank D.M. Livingston (Dana-Farber Cancer Institute) and D.L. Turner (University of Michigan) for providing DNA constructs; D. Swing for help with BAC transgenic mice and allograft experiment; W.D. Foulkes, A. Spurdle, H. Thorne, Y.C. Har, P.S. Yee, A. Saleh, the Georgetown-Lombardi Comprehensive Cancer Center, Familial Cancer and Histopathology and Tissue Share Resources, who helped with the human tumor samples; S. Burkett for cytogenetic analysis; C.H. Kim for microarray analysis, and A. Kane and R. Frederickson for illustrations. The research was sponsored by the Center for Cancer Research, US National Cancer Institute and US National Institutes of Health.
Author information
Authors and Affiliations
Consortia
Contributions
S.C. conceived the idea, conducted all the experiments and wrote the manuscript, R.-H.W. and C.-X.D. helped with xenograft experiment, provided mouse tumor samples and cell lines, K.A. carried out bioinformatics analysis, K.-A.K. helped with experiments, B.K.M. helped with mouse work, D.C.H. performed histopathological analysis, L.C. analyzed human tumor samples, M.B., P.M., M.H.G., KConFab, L.M.L., K.S.M., S.H.T., E.P., C.I. and S.W.B. provided human tumor samples, S.K.S. conceived the idea, supervised the study and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10, Supplementary Tables 1–6, Supplementary Methods and Supplementary Discussion (PDF 2307 kb)
Rights and permissions
About this article
Cite this article
Chang, S., Wang, RH., Akagi, K. et al. Tumor suppressor BRCA1 epigenetically controls oncogenic microRNA-155. Nat Med 17, 1275–1282 (2011). https://doi.org/10.1038/nm.2459
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2459
This article is cited by
-
Targeted eicosanoids profiling reveals a prostaglandin reprogramming in breast Cancer by microRNA-155
Journal of Experimental & Clinical Cancer Research (2021)
-
A comprehensive review on oncogenic miRNAs in breast cancer
Journal of Genetics (2021)
-
Discovery and function exploration of microRNA-155 as a molecular biomarker for early detection of breast cancer
Breast Cancer (2021)
-
MicroRNAs, DNA damage response and ageing
Biogerontology (2020)
-
Exosomes of pasteurized milk: potential pathogens of Western diseases
Journal of Translational Medicine (2019)