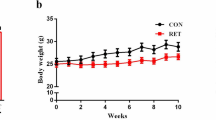
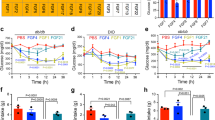
Abstract
Exercise, obesity and type 2 diabetes are associated with elevated plasma concentrations of interleukin-6 (IL-6). Glucagon-like peptide-1 (GLP-1) is a hormone that induces insulin secretion. Here we show that administration of IL-6 or elevated IL-6 concentrations in response to exercise stimulate GLP-1 secretion from intestinal L cells and pancreatic alpha cells, improving insulin secretion and glycemia. IL-6 increased GLP-1 production from alpha cells through increased proglucagon (which is encoded by GCG) and prohormone convertase 1/3 expression. In models of type 2 diabetes, the beneficial effects of IL-6 were maintained, and IL-6 neutralization resulted in further elevation of glycemia and reduced pancreatic GLP-1. Hence, IL-6 mediates crosstalk between insulin-sensitive tissues, intestinal L cells and pancreatic islets to adapt to changes in insulin demand. This previously unidentified endocrine loop implicates IL-6 in the regulation of insulin secretion and suggests that drugs modulating this loop may be useful in type 2 diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Spranger, J. et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 52, 812–817 (2003).
Herder, C. et al. Association of systemic chemokine concentrations with impaired glucose tolerance and type 2 diabetes: results from the Cooperative Health Research in the Region of Augsburg Survey S4 (KORA S4). Diabetes 54 (suppl. 2), S11–S17 (2005).
Mohamed-Ali, V. et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-α, in vivo. J. Clin. Endocrinol. Metab. 82, 4196–4200 (1997).
Lazar, M.A. How obesity causes diabetes: not a tall tale. Science 307, 373–375 (2005).
Ostrowski, K., Rohde, T., Zacho, M., Asp, S. & Pedersen, B.K. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J. Physiol. (Lond.) 508, 949–953 (1998).
Pedersen, B.K. & Febbraio, M.A. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol. Rev. 88, 1379–1406 (2008).
Febbraio, M.A. et al. Glucose ingestion attenuates interleukin-6 release from contracting skeletal muscle in humans. J. Physiol. (Lond.) 549, 607–612 (2003).
Carey, A.L. et al. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes 55, 2688–2697 (2006).
Mooney, R.A. Counterpoint: interleukin-6 does not have a beneficial role in insulin sensitivity and glucose homeostasis. J. Appl. Physiol. 102, 816–818, discussion 818–819 (2007).
Jansson, J.O. & Wallenius, V. Point-counterpoint: interleukin-6 does/does not have a beneficial role in insulin sensitivity and glucose homeostasis. J. Appl. Physiol. 102, 821 (2007).
Pedersen, B.K. & Febbraio, M.A. Point: interleukin-6 does have a beneficial role in insulin sensitivity and glucose homeostasis. J. Appl. Physiol. 102, 814–816 (2007).
Weigert, C., Lehmann, R. & Schleicher, E.D. Point-counterpoint: interleukin-6 does/does not have a beneficial role in insulin sensitivity and glucose homeostasis. J. Appl. Physiol. 102, 820–821, author reply 825 (2007).
Ellingsgaard, H. et al. Interleukin-6 regulates pancreatic alpha-cell mass expansion. Proc. Natl. Acad. Sci. USA 105, 13163–13168 (2008).
Creutzfeldt, W. The incretin concept today. Diabetologia 16, 75–85 (1979).
Kieffer, T.J. & Habener, J.F. The glucagon-like peptides. Endocr. Rev. 20, 876–913 (1999).
Lund, P.K., Goodman, R.H., Dee, P.C. & Habener, J.F. Pancreatic preproglucagon cDNA contains two glucagon-related coding sequences arranged in tandem. Proc. Natl. Acad. Sci. USA 79, 345–349 (1982).
Bell, G.I., Santerre, R.F. & Mullenbach, G.T. Hamster preproglucagon contains the sequence of glucagon and two related peptides. Nature 302, 716–718 (1983).
Rouillé, Y., Kantengwa, S., Irminger, J.C. & Halban, P.A. Role of the prohormone convertase PC3 in the processing of proglucagon to glucagon-like peptide 1. J. Biol. Chem. 272, 32810–32816 (1997).
Zhu, X. et al. Disruption of PC1/3 expression in mice causes dwarfism and multiple neuroendocrine peptide processing defects. Proc. Natl. Acad. Sci. USA 99, 10293–10298 (2002).
Rouillé, Y., Martin, S. & Steiner, D.F. Differential processing of proglucagon by the subtilisin-like prohormone convertases PC2 and PC3 to generate either glucagon or glucagon-like peptide. J. Biol. Chem. 270, 26488–26496 (1995).
Furuta, M. et al. Severe defect in proglucagon processing in islet A-cells of prohormone convertase 2 null mice. J. Biol. Chem. 276, 27197–27202 (2001).
Thyssen, S., Arany, E. & Hill, D.J. Ontogeny of regeneration of beta-cells in the neonatal rat after treatment with streptozotocin. Endocrinology 147, 2346–2356 (2006).
De León, D.D. et al. Role of endogenous glucagon-like peptide-1 in islet regeneration after partial pancreatectomy. Diabetes 52, 365–371 (2003).
Nie, Y. et al. Regulation of pancreatic PC1 and PC2 associated with increased glucagon-like peptide 1 in diabetic rats. J. Clin. Invest. 105, 955–965 (2000).
Kilimnik, G., Kim, A., Steiner, D.F., Friedman, T.C. & Hara, M. Intraislet production of GLP-1 by activation of prohormone convertase 1/3 in pancreatic alpha-cells in mouse models of ss-cell regeneration. Islets 2, 149–155 (2010).
Hansen, A.M. et al. Upregulation of alpha cell glucagon-like peptide 1 (GLP-1) in Psammomys obesus-an adaptive response to hyperglycaemia? Diabetologia 54, 1379–1387 (2011).
Anini, Y. & Brubaker, P.L. Role of leptin in the regulation of glucagon-like peptide-1 secretion. Diabetes 52, 252–259 (2003).
Ehses, J.A. et al. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes 56, 2356–2370 (2007).
Li, M. et al. Successful modulation of type 2 diabetes in db/db mice with intra-bone marrow–bone marrow transplantation plus concurrent thymic transplantation. J. Autoimmun. 35, 414–423 (2010).
Sabio, G. et al. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science 322, 1539–1543 (2008).
Wueest, S. et al. Deletion of Fas in adipocytes relieves adipose tissue inflammation and hepatic manifestations of obesity in mice. J. Clin. Invest. 120, 191–202 (2010).
Reimann, F. et al. Glucose sensing in L cells: a primary cell study. Cell Metab. 8, 532–539 (2008).
Yamaguchi, K. et al. Blockade of IL-6 signaling exacerbates liver injury and suppresses antiapoptotic gene expression in methionine choline-deficient diet-Fed db/db mice. Lab. Invest. 91, 609–618 (2011).
Ueda, S.Y. et al. Changes in gut hormone levels and negative energy balance during aerobic exercise in obese young males. J. Endocrinol. 201, 151–159 (2009).
Adam, T.C. & Westerterp-Plantenga, M.S. Activity-induced GLP-1 release in lean and obese subjects. Physiol. Behav. 83, 459–466 (2004).
Martins, C., Morgan, L.M., Bloom, S.R. & Robertson, M.D. Effects of exercise on gut peptides, energy intake and appetite. J. Endocrinol. 193, 251–258 (2007).
Chanoine, J.P. et al. GLP-1 and appetite responses to a meal in lean and overweight adolescents following exercise. Obesity (Silver Spring) 16, 202–204 (2008).
Dela, F., von Linstow, M.E., Mikines, K.J. & Galbo, H. Physical training may enhance beta-cell function in type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 287, E1024–E1031 (2004).
Delmeire, D. et al. Prior in vitro exposure to GLP-1 with or without GIP can influence the subsequent beta cell responsiveness. Biochem. Pharmacol. 68, 33–39 (2004).
Hinke, S.A., Hellemans, K. & Schuit, F.C. Plasticity of the beta cell insulin secretory competence: preparing the pancreatic beta cell for the next meal. J. Physiol. (Lond.) 558, 369–380 (2004).
Holz, G.G., Kuhtreiber, W.M. & Habener, J.F. Pancreatic beta-cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1(7–37). Nature 361, 362–365 (1993).
Di Gregorio, G.B., Hensley, L., Lu, T., Ranganathan, G. & Kern, P.A. Lipid and carbohydrate metabolism in mice with a targeted mutation in the IL-6 gene: absence of development of age-related obesity. Am. J. Physiol. Endocrinol. Metab. 287, E182–E187 (2004).
Bosco, D. et al. Unique arrangement of alpha- and beta-cells in human islets of Langerhans. Diabetes 59, 1202–1210 (2010).
Rodriguez-Diaz, R. et al. Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nat. Med. 17, 888–892 (2011).
Kieffer, T.J. et al. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology 136, 3585–3596 (1995).
Holmes, A.G. et al. Prolonged interleukin-6 administration enhances glucose tolerance and increases skeletal muscle PPARα and UCP2 expression in rats. J. Endocrinol. 198, 367–374 (2008).
Franckhauser, S. et al. Overexpression of Il6 leads to hyperinsulinaemia, liver inflammation and reduced body weight in mice. Diabetologia 51, 1306–1316 (2008).
Sadagurski, M. et al. Human IL6 enhances leptin action in mice. Diabetologia 53, 525–535 (2010).
Wallenius, V. et al. Interleukin-6-deficient mice develop mature-onset obesity. Nat. Med. 8, 75–79 (2002).
Weigert, C. et al. Direct cross-talk of interleukin-6 and insulin signal transduction via insulin receptor substrate-1 in skeletal muscle cells. J. Biol. Chem. 281, 7060–7067 (2006).
Schuler, B. et al. Optimal hematocrit for maximal exercise performance in acute and chronic erythropoietin-treated mice. Proc. Natl. Acad. Sci. USA 107, 419–423 (2010).
Jevdjovic, T. et al. Effects of insulin–like growth factor-I treatment on the endocrine pancreas of hypophysectomized rats: comparison with growth hormone replacement. Eur. J. Endocrinol. 151, 223–231 (2004).
Jevdjovic, T. et al. The effect of hypophysectomy on pancreatic islet hormone and insulin-like growth factor I content and mRNA expression in rat. Histochem. Cell Biol. 123, 179–188 (2005).
Sporeno, E. et al. Human interleukin-6 receptor super-antagonists with high potency and wide spectrum on multiple myeloma cells. Blood 87, 4510–4519 (1996).
Parnaud, G. et al. Proliferation of sorted human and rat beta cells. Diabetologia 51, 91–100 (2008).
Gribble, F.M., Williams, L., Simpson, A.K. & Reimann, F. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes 52, 1147–1154 (2003).
Rudich, A. et al. Indinavir uncovers different contributions of GLUT4 and GLUT1 towards glucose uptake in muscle and fat cells and tissues. Diabetologia 46, 649–658 (2003).
Acknowledgements
We acknowledge O. Madsen and D. Steiner for their suggestion to investigate the role of IL-6 on GLP-1 and PC1/3. We thank R. Prazak and M. Borsigova for technical assistance and M. Niessen for discussions. This work was supported by grants from the Swiss National Science Foundation and by the Merck Investigator Studies Program. J.A.E. was supported by grants from the Hartman Muller organization, the Child and Family Research Institute and the University of British Columbia and has salary support from the Canadian Diabetes Association. We obtained human islets thanks to grant 31-2008-416 from the Juvenile Diabetes Research Foundation. D.J.D. is supported by the Canada Research Chair in Regulatory Peptides, the Banting and Best Diabetes Centre–Novo Nordisk Chair in Incretin Biology and Canadian Institutes of Health Research operating grant 93749. F.M.G., F.R. and A.M.H. are supported by grants from the Wellcome Trust (WT088357 to F.M.G. and WT084210 to F.R.) and EU FP7 grant 266408.
Author information
Authors and Affiliations
Contributions
H.E. designed and performed experiments, analyzed data and wrote the manuscript. I.H., B.S., A.M.H., L.L.B., D.T.M., E.E., J.G., S.W., A.M.K.H., D.K., M.R., M.G., D.J.D., F.R. and F.M.G. performed experiments. Y.D.M. isolated human islets. K.B. and P.A.H. provided FACS-sorted human islet cells. D.J.D., F.M.G. and F.R. provided mice for this study. J.A.E. and M.Y.D. designed experiments and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Table 1 (PDF 311 kb)
Rights and permissions
About this article
Cite this article
Ellingsgaard, H., Hauselmann, I., Schuler, B. et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med 17, 1481–1489 (2011). https://doi.org/10.1038/nm.2513
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2513
This article is cited by
-
Muscle-to-organ cross-talk mediated by interleukin 6 during exercise: a review
Sport Sciences for Health (2024)
-
Relevance and consequence of chronic inflammation for obesity development
Molecular and Cellular Pediatrics (2023)
-
Glucagon-like peptide-1 and glucagon-like peptide-2 regulation during human liver regeneration
Scientific Reports (2023)
-
Beyond the pancreas: contrasting cardiometabolic actions of GIP and GLP1
Nature Reviews Endocrinology (2023)
-
Kardiovaskuläre Protektion durch Rezeptoragonisten des glukagonähnlichen Peptids 1
Die Diabetologie (2023)