Abstract
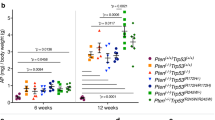
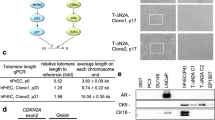
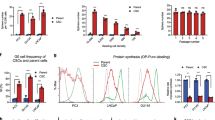
Prostate cancer mortality results from metastasis to bone and hormone-independent tumor growth. Models to study these progressive changes are lacking. Here we describe the propagation of advanced human prostate cancer by direct transfer of surgical samples from patients into immune-deficient male SCID mice. Explants from six of eight patients formed prostate tumors and two showed unique cytogenetic, biologic and molecular features that were retained through six or more passages. One grew in an androgen-independent fashion, whereas the second formed tumors that regressed following castration then regrew. Micrometastatic disease was detected in the hematopoietic tissues of half of the recipient mice. Thus selected specimens of advanced human prostate cancer can be propagated in SCID mice in a manner that recapitulates the clinical transition from androgen-sensitive to androgen-independent growth, accompanied by micrometastasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Ware, J.L. & Maygarden, S.J. Metastatic diversity in human prostatic carcinoma: Implications of growth factors and growth factor receptors for the metastatic phenotype. Pathol. Immunopathol. Res. 8, 231–249 (1989).
Pretlow, T.G., Delmoro, C.M., Dilley, G.G., Spadafora, C.G. & Pretlow, T.P. Transplantation of human prostatic carcinoma into nude mice in Matrigel. Cancer Res. 51, 3814–3817 (1991).
Pretlow, T.G. et al. Xenografts of primary human prostatic carcinoma. J. Natl. Cancer Inst. 85, 394–398 (1993).
Luberoff, D.M., Cohen, M.B., Schultz, L.D. & Beamer, W.G. Survival of human prostate carcinoma, benign hyperplastic prostate tissues, and IL-2 activated lymphocytes in scid mice. Prostate 1, 32–41 (1995).
Wainstein, M.A. et al. CWR22: Androgen-dependent xenograft model derived from a primary human prostatic carcinoma. Cancer Res. 54, 6049–6052 (1994).
Liu, A.Y., Corey, E., Bladou, F., Lange, P.H. & Vessella, R.L., Prostatic cell lineage markers: Emergence of BCL2+ cells of human prostate cancer xenograft LuCaP 23 following castration. Intl. J. Cancer 65, 85–89 (1996).
van Weerden, W. M. et al. Development of seven new human prostate tumor xenograft models and their histopathological characterization. Am. J. Pathol. 149, 1055–1062 (1996).
Wu, H.C. et al. Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: Role of bone stromal cells. Intl. J. Cancer 57, 406–412 (1994).
Noel, A., Simon, N., Raus, J. & Foidart, J.M. Basement membrane components (Matrigel) promote the tumorigenicity of human breast adenocarcinoma MCF7 cells and provide an in vivo model to assess the responsiveness of cells to estrogen. Biochemical Pharmacology 43, 1263–1267 (1992).
Lim, D.J. et al. Growth of an androgen-sensitive human prostate cancer cell line, LNCaP, in nude mice. Prostate 22, 109–118 (1993).
Pang, S. et al. Prostate tissue specificity of the prostate-specific antigen promoter isolated from a patient with prostate cancer. Hum. Gene Ther. 6, 1417–1426 (1995).
Horoszewicz, J.S. et al. LNCaP model of human prostatic carcinoma. Cancer Res. 43, 1809–1818 (1983).
Wakasugi, H. et al. Frequent development of murine T-cell lymphomas with TcR alpha/beta+ cells in BALB/c nude mice. Jpn. J. Cancer Res. 86, 1086–1096 (1995).
Rowe, M. et al. Epstein-Barr virus (EBV)-associated lymphoproliferative disease in the SCID mouse model: Implications for the pathogenesis of EBV-positive lymphomas in man. J. Exp. Med. 173, 147–158 (1991).
Taplin, M.-E. et al. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. New Engl. J. Med. 332, 1393–1398 (1995).
Gaddipati, P. et al. Frequent detection of codon 877 mutation in the androgen receptor gene in advanced prostate cancers. Cancer Res. 54, 2861–2864 (1994).
Brinkmann, A.O. et al. Androgen receptor mutations. J. Steroid Biochem. Mol. Biol. 53, 443–448 (1995).
Newmark, J.R. et al. Androgen receptor gene mutations in human prostate cancer. Proc. Natl. Acad. Sci. USA 89, 6319–6323 (1992).
Tilley, W.D., Buchanan, G., Hickey, T.E. & Bentel, J.M. Mutations in the androgen receptor gene are associated with progression of human prostate cancer to androgen independence. Clin.Cancer Res. 2, 277–285 (1996).
Veldscholte, J. et al. The androgen receptor in LNCaP cells contains the mutation in the ligand binding domain which affects steroid binding characteristics and response to antiandrogens. J. Steroid Biochem. Mol. Biol. 41, 665–669 (1992).
Sutherland, R.W. et al. Androgen receptor gene mutations are rarely associated with isolated penile hypospadias. J. Urol. 156, 828–831 (1996).
Ghossein, R.A. et al. Detection of circulating tumor cells in patients with localized and metastatic prostatic carcinoma: Clinical implications. J. Clin. Oncol. 13, 1195–1200 (1995).
Seiden, M.V. et al. Detection of circulating tumor cells in men with localized prostate cancer. J. Clin. Oncol. 12, 2634–2639 (1994).
Wood, D.P., Banks, E.R., Humphreys, S., McRoberts, J.W. & Rangnekar, V.M. Identification of bone marrow micrometastases in patients with prostate cancer. Cancer 74, 2533–2540 (1994).
Katz, A.E. et al. Molecular staging of prostate cancer with the use of an enhanced reverse transcriptase-PCR assay. Urology 43, 765–775 (1994).
Brandt, B. et al. Isolation of prostate-derived single cells and cell clusters from human peripheral blood. Cancer Res. 56, 4556–4561 (1996).
Baiocchi, R.A. & Caligiuri, M.A. Low-dose interleukin 2 prevents the development of Epstein-Barr virus (EBV)-associated lymphoproliferative disease in scid/scid mice reconstituted i.p. with EBV-seropositive human peripheral blood lymphocytes. Proc. Natl. Acad. Sci. USA 91, 5577–5581 (1994).
Brothman, A.R., Peehl, D.M., Patel, A.M. & McNeal, J.E. Frequency and pattern of karyotypic abnormalities in human prostate cancer. Cancer Res. 50, 3795–3803 (1990).
Debruyne, F.M. et al. Cytogenetics of prostate cancer. Consensus Conference on Diagnosis and Prognostic Parameters in Localized Prostate Cancer. Scand. J. Urol. Nephrol. 162, 65-71-115-27 (1993).
Micale, M.A., Sanford, J.S., Powell, I. J., Sakr, W.A. & Wolman, S.R. Defining the ex tend and nature of cytogenetic events in prostatic adenocarcinoma: Paraffin FISH vs. metaphase analysis. Cancer Gen. Cytogen. 69, 7–12 (1993).
Cher, M.L. et al. Genetic alterations in untreated metastases and androgen-independent prostate cancer detected by comparative genomic hybridization and allelotyping. Cancer Res. 56, 3091–3102 (1996).
Cooney, K.A. et al. Distinct regions of allelic loss on 13q in prostate cancer. Cancer Res. 56, 1142–1145 (1996).
Gleave, M.E., Hsieh, J.T., Wu, H.C., von Eschenbach, A.C. & Chung, L. W.K. Serum prostate specific antigen levels in mice bearing human prostate LNCaP tumors are determined by tumor volume and endocrine and growth factors. Cancer Res. 52, 1598–1605 (1992).
Nagabhushan, M. et al. CWR22: the first human prostate cancer xenograft with strongly androgen-dependent and relapsed strains both in vivo and in soft agar. Cancer Res. 56, 3042–3046 (1996).
Marcelli, M., Haidacher, S.J., Plymate, S.R. & Birnbaum, R.S. Altered growth and insulin-like growth factor-binding protein-3 production in PC3 prostate carcinoma cells stably transfected with a constitutively active androgen receptor complemen tary deoxyribonucleic acid. Endocrinology 136, 1040–1048 (1995).
Umekita, Y., Hiipakka, R.A., Kokontis, J.M. & Shutsung, L. Human prostate tumor growth in athymic mice: inhibition by androgens and stimulation by finasteride. Proc. Natl. Acad. Sci.USA 93, 11802–11807 (1996).
Shtivelman, E. & Namikawa, R. Species-specific metastasis of human tumor cells in the severe combined immunodeficiency mouse engrafted with human tissue. Proc. Natl. Acad. Sci. USA 92, 4661–4665 (1995).
Aldrovandi, G.M. et al. The SCID-hu mouse as a model for HIV-1 infection. Nature 363, 732–736 (1993).
Sambrook, J., Fritsch, E.F. & Maniatis, T. Molecular Cloning: A Laboratory Manual, edn.2 (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 1989).
Saiki, R.K. et al. Enzymatic amplification of β-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science 230, 1350–1354 (1985).
Hsu, S.M., Raine, L. & Fanger, H. A comparative study of the peroxidase-antiperoxidase method and an avidin-biotin complex method for studying polypeptide hormones with radioimmunoassay antibodies. Am. J. Clin. Pathol. 75, 734–738 (1981).
Marcelli, M. et al. Definition of the human androgen receptor gene structure permits the identification of mutations that cause androgen resistance: Premature termination of the receptor protein at amino acid residue 588 causes complete androgen resistance. Mol. Endocrinol. 90, 1105–1116 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Klein, K., Reiter, R., Redula, J. et al. Progression of metastatic human prostate cancer to androgen independence in immunodeficient SCID mice. Nat Med 3, 402–408 (1997). https://doi.org/10.1038/nm0497-402
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm0497-402