Abstract
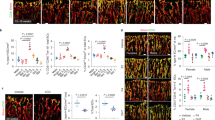
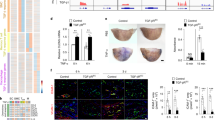
The atheroprotective effects of estrogen in women are well recognized1, but the underlying mechanisms responsible are not well understood. Blood vessel cells express the classic estrogen receptor, ERα (ref. 2—6), and are directly affected by estrogen, which inhibits the development of atherosclerotic and injury-induced vascular lesions78. We have generated mice in which the ERα gene is disrupted9 and have used a mouse model of carotid arterial injury to compare the effects of estrogen on wild-type and estrogen receptor-deficient mice. Increases in vascular medial area and smooth muscle cell proliferation were quantified following vascular injury in ovariectomized mice treated with vehicle or with physiologic levels of 17β-estradiol. Suprisingly, in both wild-type and estrogen receptor-deficient mice, 17β-estradiol markedly inhibited to the same degree all measures of vascular injury. These data demonstrate that estrogen inhibits vascular injury by a novel mechanism that is independent of the classic estrogen receptor, ERα.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Grady, D. et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann. Intern. Med. 117, 1016–1037 (1992).
Colbum, P. & Buonassis, V. Estrogen-binding sites in endothelial Cell cultures. Science 201, 817–319 (1978).
Orimo, A. et al. Vascular smooth muscle Cells as target for estrogen. Biochem. Biophys. Res. Commun. 195, 730–736 (1993).
Losordo, D.W., Kearney, M., Kim, E.A., Jekanowski, J. & Isner, J.M. Variable expression of the estrogen receptor in normal and atherosclerotic coronary arteries of premenopausal women. Circulation 89, 1501–1510 (1994).
Karas, R.H., Patterson, B.L. & Mendelsohn, M.E. Human vascular smooth muscle Cells contain functional estrogen receptor. Circulation 89, 1943–1950 (1994).
Venkov, C.D., Rankin, A.B. & Vaughan, D.E. Identification of authentic estrogen receptor in cultured endothelial Cells: A potential mechanism for steroid hormone regulation of endothelial function. Circulation 94, 727–733 (1996).
Mendelsohn, M.E. & Karas, R.H. Estrogen and the blood vessel wall. Curr. Opin. Cardial. 9, 619–626 (1994).
Farhat, M.Y., Lavigne, M.C. & Ramwell, P.W. The vascular protective effects of estrogen. FASEB J. 10, 615–624 (1996).
Lubahn, D.B. et al. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc. Natl. Acad. Sci. USA 90, 11162–11166 (1993).
Sullivan, T.R. Jr. et al. Estrogen inhibits the response-to-injury in a mouse carotid artery model. J. Clin. Invest. 96, 2482–2488 (1995).
Green, S. et al. Human oestrogen receptor cDNA: Sequence, expression and homology to verb-A. Nature 320, 134–139 (1986).
Evans, R.M. The steroid and thyroid hormone receptor superfamily. Science 240, 889–895 (1988).
Horwitz, K.B. & Horwitz, L.D. Canine vascular tissues are targets for androgens, estrogens, progestins, and glucocorticoids. J. Clin. Invest. 69, 750–759 (1982).
Koike, H., Karas, R.H., Baur, W.E., O'Donnell, T.F. Jr. & Mendelsohn, M.E. Differential-display polymerase chain reaction identifies nucleophosmin as an estrogen-regulated gene in human vascular smooth muscle Cells. J. Vasc Surg. 23, 477–482 (1996).
Kuiper, G.G.J.M., Enmark, E., Pelto-Huikko, M., Nilsson, S. & Gustafsson, J.Å. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc. Notl. Acad. Sci. USA 93, 5925–5930 (1996).
Mosselman, S., Polman, J. & Dijkema, R., ERβIdentification and characterization of a novel human estrogen receptor. FEBS Lett. 392, 49–53 (1996).
Couse, J.F. et al. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol. Endocrinol. 9, 1441–1454 (1995).
Lindner, V., Fingerle, J. & Reidy, M.A. Mouse model of arterial injury. Circ. Res. 73, 792–796 (1993).
Harder, D.R. & Coulson, P.B. Estrogen receptors and effects of estrogen on membrane electrical properties of coronary vascular smooth muscle. J. Cell. Physiol. 100, 375–382 (1979).
Jiang, C., Sarrel, P.M., Lindsey, D.C., Poole-Wilson, P.A. & Collins, P. Endothelium-independent relaxation of rabbit coronary artery by 17β-oestradiol in vitro. Br. J. Pharmacol. 104, 1033–1037 (1991).
Zhang, F., Ram, J.L., Standley, P.R. & Sowers, J.R., 17β-Estradiol attenuates voltage-dependent Ca2+ currents in A7r5 vascular smooth muscle Cell line. Am. J. Physiol. 266, C975–C980 (1994).
Williams, J.K., Adams, M.R., Herrington, D.M. & Clarkson, T.B. Short-term administration of estrogen and vascular responses of atherosclerotic coronary arteries. J. Am. Coll. Cardiol. 20, 452–457 (1992).
White, R.E., Darkow, D.J. & Lang, J.L.F. Estrogen relaxes coronary arteries by opening BKca, channels through a cGMP-dependent mechanism. Circ. Res. 77, 936–942 (1995).
Amal, J.F. et al. Ethinylestradiol does not enhance the expression of nitric oxide synthase in bovine endothelial Cells but increases the release of bioactive nitric oxide by inhibiting superoxide anion production. Proc. Natl. Acad. Sci. USA 93, 4108–4113 (1996).
Touchette, N. Man bites dogma: A new role for steroid hormones. J. NIH Res. 2, 71–74 (1990).
Graber, R., Surnida, C., Vallette, G., & Nunez, E.A. . Rapid and long-term effects of 17β-estradiol on PIP2-phospholipase C-specific activity of MCF-7 Cells. Cell. Signal. 5, 181–186 (1993).
Aronica, S.M., Kraus, W.L. & Katzenellenbogen, B.S. Estrogen action via the cAMP signaling pathway: Stimulation of adenylate cyclase and cAMP-regulated gene transcription. Proc. Natl. Acad. Sci. USA 91, 8517–8521 (1994).
Wehling, M., Neylon, C.B., Fullerton, M., Bobik, A. & Funder, J.W. Nongenomic effects of aldosterone on intraCellular Ca2+ in vascular smooth muscle Cells. Circ. Res. 76, 973–979 (1995).
Rosen, L.B., Ginty, D.D. & Greenberg, M.E. Calcium regulation of gene expression. Adv. Second Messenger Phosphoprotein Res. 30 225–253 (1995).
Brindle, P.K. & Montminy, M.R. The CREB family of transcription activators. Curr. Opin. Genet. Dev. 2, 199–204 (1992).
Fischer-Dzoga, K., Wissler, R. & Vesselinovitch, D. The effect of estradiol on the proliferation of rabbit aortic medial tissue Cells induced by hyperlipemic serum. Exp. Mol. Pathol. 39, 355–363 (1983).
Farhat, M.Y., Vargas, R., Dingaan, B. & Ramwell, P.W. In vitro effect of oestradiol on thymidine uptake in pulmonary vascular smooth muscle Cell: Role of the endothelium. Br. J. Pharmacol. 107, 679–683 (1992).
Moraghan, T. et al. Differential response in Cell proliferation to beta estradiol in coronary arterial vascular smooth muscle Cells obtained from mature female versus male animals. Endocrinology 137, 5174–5177 (1996).
Kolodgie, F.D. et al. Estradiol attenuates directed migration of vascular smooth muscle Cells in vitro. Am. J. Pathol. 148, 969–976 (1996).
Johns, A., Freay, A.D., Fraser, W., Korach, K.S. & Rubanyi, G.M. Disruption of estrogen receptor gene prevents 17β estradiol-induced angiogenesis in transgenic mice. Endocrinology 137, 4511–4513 (1996).
Adams, M.R. et al. Inhibition of coronary artery atherosclerosis by 17-beta estradiol in ovariectomized monkeys: Lack of an effect of added progesterone. Arteriosclerosis 10, 1051–1057 (1990).
Haarbo, J., Leth-Espensen, P., Strander, S. & Cristiansen, C. Estrogen monotherapy and combined estrogen progestogen replacement therapy attenuate aortic accumulation of cholesterol in ovariectomized cholesterol-fed rabbits. J. Clin. Invest. 87, 1274–1279 (1991).
Chen, S.J., Li, H.B., Durand, J., Oparil, S. & Chen, Y.F. Estrogen reduces myointimal proliferation after balloon injury of rat carotid artery. Circulation 93, 577–584 (1996).
Bourassa, P.A.K., Milos, P.M., Gaynor, B.J., Breslow, J.L. & Aiello, R.J. Estrogen reduces atherosclerotic lesion development in apolipoprotein E-deficient mice. Proc. Natl. Acad. Sci. USA 93, 10022–10027 (1996).
Chang, W.C., Nakao, J., Orimo, H. & Murota, S.I. Stimulation of prostaglandin cyclooxygenase and prostacyclin synthetase activities by estradiol in rat aortic smooth muscle Cells. Biochim. Biophys. Ada 620, 472–482 (1980).
Hayashi, T., Fukuto, J.M., Ignarro, L.J. & Chaudhuri, G. Basal release of nitric oxide from aortic rings is greater in female rabbits than in male rabbits: Implications for atherosclerosis. Proc. Natl. Acad. Sci. USA 89, 11259–11263 (1992).
Weiner, C.P. et al. Induction of calcium-dependent nitric oxide synthases by sex hormones. Proc. Natl. Acad. Sci. USA 91, 5212–5216 (1994).
Caulin-Glaser, T., Watson, G.A., Pardi, R. & Bender, J.R. Effects of 17b-estradiol on cytokine-induced endothelial Cell adhesion molecular expression. J. Clin. Invest. 98, 36–42 (1996).
White, R., Lees, J.A., Needham, M., Ham, J. & Parker, M. Structural organization and expression of the mouse estrogen receptor. Mol. Endocrinol. 1, 735–744 (1987).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Iafrati, M., Karas, R., Aronovitz, M. et al. Estrogen inhibits the vascular injury response in estrogen receptor α-deficient mice. Nat Med 3, 545–548 (1997). https://doi.org/10.1038/nm0597-545
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm0597-545
This article is cited by
-
Soybean isoflavones potentially prevent sarcopenia: a systematic review
Journal of Ethnic Foods (2023)
-
Estrogen modulates serotonin effects on vasoconstriction through Src inhibition
Experimental & Molecular Medicine (2018)
-
Exogenous 17-β estradiol administration blunts progression of established angiotensin II-induced abdominal aortic aneurysms in female ovariectomized mice
Biology of Sex Differences (2015)
-
Understanding the Role of Sex in Heart Valve and Major Vascular Diseases
Cardiovascular Engineering and Technology (2015)
-
Oestrogen signalling in white adipose progenitor cells inhibits differentiation into brown adipose and smooth muscle cells
Nature Communications (2014)