Abstract
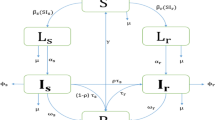
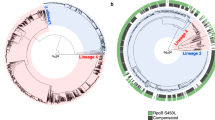
Mathematical models have recently been used to predict the future burden of multidrug-resistant tuberculosis (MDRTB)1,2,3. These models suggest the threat of multidrug resistance to TB control will depend on the relative 'fitness' of MDR strains and imply that if the average fitness of MDR strains is considerably less than that of drug-sensitive strains, the emergence of resistance will not jeopardize the success of tuberculosis control efforts. Multidrug resistance in M. tuberculosis is conferred by the sequential acquisition of a number of different single-locus mutations that have been shown to have heterogeneous phenotypic effects. Here we model the impact of initial fitness estimates on the emergence of MDRTB assuming that the relative fitness of MDR strains is heterogeneous. We find that even when the average relative fitness of MDR strains is low and a well-functioning control program is in place, a small subpopulation of a relatively fit MDR strain may eventually outcompete both the drug-sensitive strains and the less fit MDR strains. These results imply that current epidemiological measures and short-term trends in the burden of MDRTB do not provide evidence that MDRTB strains can be contained in the absence of specific efforts to limit transmission from those with MDR disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Blower, S.M. & Gerberding, J.L. Understanding, predicting and controlling the emergence of drug- resistant tuberculosis: a theoretical framework. J. Mol. Med. 76, 624–636 (1998).
Dye, C. & Williams, B.G. Criteria for the control of drug-resistant tuberculosis. Proc. Natl. Acad. Sci. USA 97, 8180–8185 (2000).
Dye, C. & Espinal, M.A. Will tuberculosis become resistant to all antibiotics? Proc. R. Soc. Lond. B. Biol. Sci. 268, 45–52 (2001).
World Health Organization. Anti-tuberculosis drug resistance in the world, report #3 (WHO, Geneva, 2004).
Andersson, D.I. & Levin, B.R. The biological cost of antibiotic resistance. Curr. Opin. Microbiol. 2, 489–493 (1999).
Anderson, R.M. & May, R.M. Infectious Diseases of Humans: Dynamics and Control. (Oxford University Press, Oxford, 1991).
Grenfell, B.T. & Anderson, R.M. Pertussis in England and Wales: an investigation of transmission dynamics and control by mass vaccination. Proc. R. Soc. Lond. B Biol. Sci. 22, 213–252 (1989).
Blower, S.M., Small, P.M. & Hopewell, P.C. Control strategies for tuberculosis epidemics: new models for old problems. Science 273, 497–500 (1996).
Blower, S.M. et al. The intrinsic transmission dynamics of tuberculosis epidemics. Nat. Med. 1, 815–821 (1995).
Vynnycky, E. & Fine, P.E. The natural history of tuberculosis: the implications of age-dependent risks of disease and the role of reinfection. Epidemiol. Infect. 119, 183–201 (1997).
Dye, C., Garnett, G.P., Sleeman, K. & Williams, B.G. Prospects for worldwide tuberculosis control under the WHO DOTS strategy: Directly observed short-course therapy. Lancet 352, 1886–1891 (1998).
Castillo-Chavez, C. & Feng, Z. To treat or not to treat: the case of tuberculosis. J. Math. Biol. 35, 629–656 (1997).
Schrag, S.J., Perrot, V. & Levin, B.R. Adaptation to the fitness costs of antibiotic resistance in Escherichia coli. Proc. R. Soc. Lond. B Biol. Sci. 264, 1287–1291 (1997).
Lipsitch, M. & Levin, B.R. The population dynamics of antimicrobial chemotherapy. Antimicrob. Agents Chemother. 41, 363–373 (1997).
Ordway, D.J., Sonnenberg, M.G., Donahue, S.A., Belisle, J.T. & Orme, I.M. Drug-resistant strains of Mycobacterium tuberculosis exhibit a range of virulence for mice. Infect. Immun. 63, 741–743 (1995).
Sherman, D.R. et al. Compensatory ahpC gene expression in isoniazid-resistant Mycobacterium tuberculosis. Science 272, 1641–1643 (1996).
Garcia-Garcia, M.L. et al. Clinical consequences and transmissibility of drug-resistant tuberculosis in southern Mexico. Arch. Intern. Med. 160, 630–636 (2000).
Teixeira, L. et al. Infection and disease among household contacts of patients with multidrug-resistant tuberculosis. Int. J. Tuberc. Lung Dis. 5, 321–328 (2001).
Cohen, T., Sommers, B. & Murray, M. The effect of drug resistance on the fitness of Mycobacterium tuberculosis. Lancet Infect. Dis. 3, 13–21 (2003).
Khatri, G.R. & Frieden, T.R. Controlling tuberculosis in India. N. Engl. J. Med. 347, 1420–1425 (2002).
World Health Organization. Treatment of tuberculosis. Guidelines for national programmes. WHO report (WHO/CDS/TB/97.220, Geneva, 1997).
Espinal, M.A. et al. Standard short-course chemotherapy for drug-resistant tuberculosis: treatment outcomes in 6 countries. JAMA 283, 2537–2545 (2000).
Styblo, K. Epidemiology of Tuberculosis: Selected Papers (Royal Netherlands Tuberculosis Association, The Hague, 1991).
Dye, C., Scheele, S., Dolin, P., Pathania, V. & Raviglione, M.C. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 282, 677–686 (1999).
Espinal, M.A. et al. Global trends in resistance to antituberculosis drugs. World Health Organization-International Union against Tuberculosis and Lung Disease Working Group on Anti-Tuberculosis Drug Resistance Surveillance. N. Engl. J. Med. 344, 1294–1303 (2001).
Verver, S. et al. Transmission of tuberculosis in a high incidence urban community in South Africa. Int. J. Epidemiol. 33, 351–357 (2004).
Small, P.M. et al. The epidemiology of tuberculosis in San Francisco: A population-based study using conventional and molecular methods. N. Engl. J. Med. 330, 1703–1709 (1994).
Murray, C.J. & Salomon, J.A. Modeling the impact of global tuberculosis control strategies. Proc. Natl. Acad. Sci. USA 95, 13881–13886 (1998).
Farmer, P. & Kim, J.Y. Community based approaches to the control of multidrug resistant tuberculosis: introducing “DOTS-plus”. BMJ 317, 671–674 (1998).
van Rie, A. et al. Exogenous reinfection as a cause of recurrent tuberculosis after curative treatment. N. Engl. J. Med. 341, 1174–1179 (1999).
Acknowledgements
We thank M. Lipsitch, B. Cooper, M. Becerra and M. Smurzynski for critical reviews of a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Cohen, T., Murray, M. Modeling epidemics of multidrug-resistant M. tuberculosis of heterogeneous fitness. Nat Med 10, 1117–1121 (2004). https://doi.org/10.1038/nm1110
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1110
This article is cited by
-
Compensatory evolution in NusG improves fitness of drug-resistant M. tuberculosis
Nature (2024)
-
The relative transmission fitness of multidrug-resistant Mycobacterium tuberculosis in a drug resistance hotspot
Nature Communications (2023)
-
Estimating tuberculosis drug resistance amplification rates in high-burden settings
BMC Infectious Diseases (2022)
-
The epidemiologic impact and cost-effectiveness of new tuberculosis vaccines on multidrug-resistant tuberculosis in India and China
BMC Medicine (2021)
-
New Developments and Insights in the Improvement of Mycobacterium tuberculosis Vaccines and Diagnostics Within the End TB Strategy
Current Epidemiology Reports (2021)