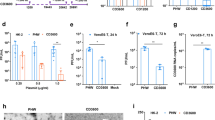
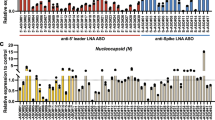
Abstract
Respiratory syncytial virus (RSV) infection is one of the major causes of respiratory tract infection for which no vaccine or antiviral treatment is available. The RSV NS1 protein seems to antagonize the host interferon (IFN) response; however, its mechanism is unknown. Here, we used a plasmid-borne small interfering RNA targeting the NS1 gene (siNS1) to examine the role of NS1 in modulating RSV infection. RSV replication was reduced in A549 cells, but not IFN–deficient Vero cells, transfected with siNS1. siNS1 induced upregulated expression of IFN-β and IFN-inducible genes in A549 cells. siNS1-transfected human dendritic cells, upon RSV infection, produced elevated type-1 IFN and induced differentiation of naive CD4+ T cells to T helper type 1 (TH1) cells. Mice treated intranasally with siNS1 nanoparticles before or after infection with RSV showed substantially decreased virus titers in the lung and decreased inflammation and airway reactivity compared to controls. Thus, siNS1 nanoparticles may provide an effective inhibition of RSV infection in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sigurs, N., Bjarnason, R., Sigurbergsson, F. & Kjellman, B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am. J. Respir. Crit. Care Med. 161, 1501–1507 (2000).
Sigurs, N., Bjarnason, R., Sigurbergsson, F., Kjellman, B. & Bjorksten, B. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics 95, 500–505 (1995).
Shay, D.K. et al. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA 282, 1440–1446 (1999).
Hall, C.B., Long, C.E. & Schnabel, K.C. Respiratory syncytial virus infections in previously healthy working adults. Clin. Infect. Dis. 33, 792–796 (2001).
Thompson, W.W. et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289, 179–186 (2003).
Sly, P.D. & Hibbert, M.E. Childhood asthma following hospitalization with acute viral bronchiolitis in infancy. Pediatr. Pulmonol. 7, 153–158 (1989).
Brandenburg, A.H., Neijens, H.J. & Osterhaus, A.D. Pathogenesis of RSV lower respiratory tract infection: implications for vaccine development. Vaccine 19, 2769–2782 (2001).
Coffin, S.E. & Offit, P.A. New vaccines against mucosal pathogens: rotavirus and respiratory syncytial virus. Adv. Pediatr. Infect. Dis. 13, 333–348 (1997).
Handforth, J., Friedland, J.S. & Sharland, M. Basic epidemiology and immunopathology of RSV in children. Paediatr. Respir. Rev. 1, 210–214 (2000).
Welliver, R.C. Review of epidemiology and clinical risk factors for severe respiratory syncytial virus (RSV) infection. J. Pediatr. 143, S112–S117 (2003).
Collins, P.L., Chanock, R.M. & Murphy, B.R. in Fields Virology Vol. 1, 4th edn (eds. Knipe, D.M., Howley, P.M. & Griffin, D.E.), 1443–1485 (Lippincott-Raven, Philadelphia, 2001).
Jin, H. et al. Recombinant respiratory syncytial viruses with deletions in the NS1, NS2, SH, and M2-2 genes are attenuated in vitro and in vivo. Virology 273, 210–208 (2000).
Teng, M. N, & Collins, P.L. Altered growth characteristics of recombinant respiratory syncytial viruses which do not produce NS2 protein. J. Virol. 73, 466–473 (1999).
Teng, M.N. et al. Recombinant respiratory syncytial virus that does not express the NS1 or M2-2 protein is highly attenuated and immunogenic in chimpanzees. J. Virol. 74, 9317–9321 (2000).
Murphy, B.R & Collins, P.L. Live-attenuated virus vaccines for respiratory syncytial and parainfluenza viruses: applications of reverse genetics. J. Clin. Invest. 110, 21-27 (2002).
Hall, C.B., Walsh, E.E., Long, C.E. & Schnabel, K.C. Immunity to and frequency of reinfection with respiratory syncytial virus. J. Infect. Dis. 163, 693–698 (1991).
Roman, M. et al. Respiratory syncytial virus infection in infants is associated with predominant TH2-like response. Am. J. Respir. Crit. Care Med. 156, 190–195 (1997).
Matsuse, H. et al. Recurrent respiratory syncytial virus infections in allergen-sensitized mice lead to persistent airway inflammation and hyperresponsiveness. J. Immunol. 164, 6583–6592 (2000).
Behera, A.K., Kumar, M., Lockey, R.F. & Mohapatra, S.S. Adenovirus-mediated interferon gamma gene therapy for allergic asthma: involvement of interleukin 12 and STAT4 signaling. Hum. Gene Ther. 13, 1697–1709 (2002).
Kumar, M., Behera, A.K., Matsuse, H., Lockey, R.F. & Mohapatra, S.S. Intranasal IFN-γ gene transfer protects BALB/c mice against respiratory syncytial virus infection. Vaccine 18, 558–567 (1999).
Kumar, M. et al. Intranasal gene transfer by chitosan-DNA nanospheres protects BALB/C mice against acute respiratory syncytial virus infection. Hum. Gene Ther. 13, 1415–1425 (2002).
Kumar, M. et al. Chitosan IFN-g-pDNA nanoparticle (CIN) therapy for allergic asthma. Genetic Vaccines and Ther. 1, 3–12 (2003).
Mohapatra, SS. Mucosal gene expression vaccine: a novel a vaccine strategy for respiratory syncytial virus. Pediatr. Infect. Dis J. 22, S100–S103 (2003).
Hellermann, G., Mohapatra, SS. Genetic Therapy: on the brink of a newfuture. Genetic Vaccines and Ther. 1, 1–3 (2003).
Bossert, B. & Conzelmann, K.K. Respiratory syncytial virus (RSV) nonstructural (NS) proteins as host range determinants: a chimeric bovine RSV with NS genes from human RSV is attenuated in interferon-competent bovine cells. J. Virol. 76, 4287–4293 (2002).
Bossert, B., Marozin, S, & Conzelmann, K.K. Nonstructural proteins NS1 and NS2 of bovine respiratory syncytial virus block activation of interferon regulatory factor 3. J. Virol. 77, 8661–8668 (2003).
Schlender, J., Bossert, B., Buchholz, U & Conzelmann, K.K. Bovine respiratory syncytial virus nonstructural proteins NS1 and NS2 cooperatively antagonize α/β interferon-induced antiviral response. J. Virol. 74, 8234–8242 (2000).
Spann, K.M., Tran, K.C., Chi, B., Rabin, R.L. & Collins, P.L. Suppression of the induction of α, β, and γ interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages. J. Virol. 78, 4363–4369 (2004).
Fire, A. RNA-triggered gene silencing. Trends Genet. 15, 358–363 (1999).
Zhang, W., Singam, R., Hellermann, G., Kong, X., San Juan, H., Lockey, R.F., Wu, S.J., Porter, K., Mohapatra, S.S. Attenuation of dengue virus infection by adeno- associated virus-mediated siRNA delivery. Genetic Vaccines Ther. 2, 8–12 (2004).
Hallak, L.K., Collins, P.L., Knudson, W. & Peeples, M.E. Iduronic acid-containing glycosaminoglycans on target cells are required for efficient respiratory syncytial virus infection. Virology 271, 264–275 (2000).
Mosca, J.D. & Pitha, P.M. Transcriptional and posttranscriptional regulation of exogenous human β interferon gene in simian cells defective in interferon synthesis. Mol. Cell. Biol. 6, 2279–2283 (1986).
Leaman, D.W. et al. Targeted therapy of respiratory syncytial virus in African green monkeys by intranasally administered 2-5A antisense. Virology 292, 70–77 (2002).
Fisher, T.L., Terhorst, T., Cao, X. & Wagner, R.W. Intracellular disposition and metabolism of fluorescently-labeled unmodified and modified oligonucleotides microinjected into mammalian cells. Nucleic Acids Res. 21, 3857–3865 (1993)
Kole, R. & Sazani, P. Antisense effects in the cell nucleus: modification of splicing. Curr. Opin. Mol. Ther. 3, 229–234 (2001).
Billy, E., Brondani, V., Zhang, H., Muller, U. & Filipowicz, W. Specific interference with gene expression induced by long, double-stranded RNA in mouse embryonal teratocarcinoma cell lines. Proc. Natl. Acad. Sci. USA 98, 14428–14433 (2001).
Arnold, R., Humbert, B., Werchau, H., Gallati, H. & Konig, W. Interleukin-8, interleukin-6, and soluble tumor necrosis factor receptor type-I release from a human pulmonary epithelial cell line (A549) exposed to respiratory syncytial virus. Immunology 82, 126–133 (1994).
Atreya, P.L., Peeples, M.E. & Collins, P.L. The NS1 protein of human respiratory syncytial virus is a potent inhibitor of minigenome transcription and RNA replication. J. Virol. 72, 1452–1461 (1998).
Stark, G.R., Kerr, I.M., Williams, B.R., Silverman, R.H. & Schreiber, R.D. How cells respond to interferons. Annu. Rev. Biochem. 67, 227–264 (1998).
Barnes, B., Lubyova, B. & Pitha, P.M. On the role of IRF in host defense. J. Interferon Cytokine Res. 22, 59–71 (2002).
Harada, H. et al. Structurally similar but functionally distinct factors, IRF-1 and IRF-2, bind to the same regulatory elements of IFN and IFN-inducible genes. Cell 58, 729–739 (1989).
Pine, R., Decker, T., Kessler, D.S., Levy, D.E. & Darnell, JE Jr. Purification and cloning of interferon-stimulated gene factor 2 (ISGF2): ISGF2 (IRF-1) can bind to the promoters of both beta interferon- and interferon-stimulated genes but is not a primary transcriptional activator of either. Mol. Cell. Biol. 10, 2448–2457 (1990).
Sledz, C.A., Holko, M., de Veer, M.J., Silverman, R.H. & Williams, B.R. Activation of the interferon system by short-interfering RNAs. Nat. Cell Biol. 5, 834–839 (2003).
Bridge, A.J., Pebernard, S., Ducraux, A., Nicoulaz, A.L. & Iggo, R. Induction of an interferon response by RNAi vectors in mammalian cells. Nat. Genet. 34, 263–264 (2003).
Sato, Y. et al. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science 273, 352–354 (1996).
Pebernard, S. & Iggo, R.D. Determinants of interferon-stimulated gene induction by RNAi vectors. Differentiation 72, 103–111 (2004).
Bont, L., Kavelaars, A., Heijnen, C.J., van Vught, A.J. & Kimpen, J.L. Monocyte interleukin-12 production is inversely related to duration of respiratory failure in respiratory syncytial virus bronchiolitis. J. Infect. Dis. 181, 1772–1775 (2000).
Bartz, H. et al. Respiratory syncytial virus decreases the capacity of myeloid dendritic cells to induce interferon-gamma in naive T cells. Immunology 109, 49–57 (2003).
Acknowledgements
We thank M.E. Peeples (Columbus Children's Research Institute, Ohio) for his rgRSV stock, O. Haller (Freiburg University, Germany) for his gift of MxA antibody, Saneron-Cell Therapeutics (Tampa, Florida) for the cord blood cells and Moffitt Flow Cytometry and Microarray Core for their assistance. SSM is supported by the grants from the Veterans' Affairs Merit Review Award and US National Institutes of Health #5RO1HL71101-01A2. The support from the Joy McCann Culverhouse Endowment to the Division of Allergy and Immunology, Department of Internal Medicine, College of Medicine, and the Veterans' Affairs Hospital is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Weidong Zhang and Shyam S. Mohapatra have filed a patent application relating to siNS1. Shyam S. Mohapatra is a scientific founder and advisor to Transgenex Nanobiotech Inc.
Rights and permissions
About this article
Cite this article
Zhang, W., Yang, H., Kong, X. et al. Inhibition of respiratory syncytial virus infection with intranasal siRNA nanoparticles targeting the viral NS1 gene. Nat Med 11, 56–62 (2005). https://doi.org/10.1038/nm1174
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1174
This article is cited by
-
Selective targeting of MYC mRNA by stabilized antisense oligonucleotides
Oncogene (2021)
-
Revisiting respiratory syncytial virus’s interaction with host immunity, towards novel therapeutics
Cellular and Molecular Life Sciences (2020)
-
Application of Locked Nucleic Acid Oligonucleotides for siRNA Preclinical Bioanalytics
Scientific Reports (2019)
-
RNA interference-based therapy and its delivery systems
Cancer and Metastasis Reviews (2018)
-
Polysaccharide-based Nanoparticles for Gene Delivery
Topics in Current Chemistry (2017)