Abstract
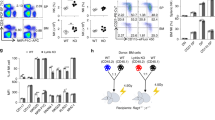
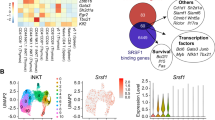
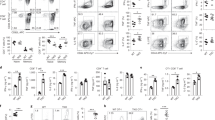
The adaptor molecule SAP is expressed in T lymphocytes and natural killer (NK) cells, where it regulates cytokine production and cytotoxicity1,2,3. Here, we show that SAP, encoded by the SH2D1A gene locus, also has a crucial role during the development of NKT cells, a lymphocyte subset with immunoregulatory functions in response to infection, cancer and autoimmune disease4. Following stimulation with the NKT cell–specific agonist α-galactosyl ceramide (αGC), Sh2d1a−/− splenocytes did not produce cytokines or activate other lymphoid lineages in an NKT cell–dependent manner. While evaluating the abnormalities in αGC-induced immune responses, we observed that Sh2d1a−/− animals lacked NKT cells in the thymus and peripheral organs. The defect in NKT cell ontogeny was hematopoietic cell autonomous and could be rescued by reconstitution of SAP expression within Sh2d1a−/− bone marrow cells. Seventeen individuals with X-linked lymphoproliferative disease (XLP), who harbored germline mutations in SH2D1A, also lacked NKT cells. Furthermore, a female XLP carrier showed completely skewed X chromosome inactivation within NKT cells, but not T or B cells. Thus, SAP is a crucial regulator of NKT cell ontogeny in humans and in mice. The absence of NKT cells may contribute to the phenotypes of SAP deficiency, including abnormal antiviral and antitumor immunity and hypogammaglobulinemia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Latour, S. & Veillette, A. Molecular and immunological basis of X-linked lymphoproliferative disease. Immunol. Rev. 192, 212–224 (2003).
Sharifi, R. et al. SAP mediates specific cytotoxic T-cell functions in X-linked lymphoproliferative disease. Blood 103, 3821–3827 (2004).
Cannons, J.L. et al. SAP regulates TH2 differentiation and PKC-θ-mediated activation of NF-κB1. Immunity 21, 693–706 (2004).
Kronenberg, M. & Gapin, L. The unconventional lifestyle of NKT cells. Nat. Rev. Immunol. 2, 557–568 (2002).
Coffey, A.J. et al. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat. Genet. 20, 129–135 (1998).
Nichols, K.E. et al. Inactivating mutations in an SH2 domain-encoding gene in X-linked lymphoproliferative syndrome. Proc. Natl. Acad. Sci. USA 95, 13765–13770 (1998).
Sayos, J. et al. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature 395, 462–469 (1998).
Schuster, V. & Kreth, H.W. X-linked lymphoproliferative disease is caused by deficiency of a novel SH2 domain-containing signal transduction adaptor protein. Immunol. Rev. 178, 21–28 (2000).
Wu, C. et al. SAP controls T cell responses to virus and terminal differentiation of TH2 cells. Nat. Immunol. 2, 410–414 (2001).
Czar, M.J. et al. Altered lymphocyte responses and cytokine production in mice deficient in the X-linked lymphoproliferative disease gene SH2D1A/DSHP/SAP. Proc. Natl. Acad. Sci. USA 98, 7449–7454 (2001).
Malbran, A. et al. Loss of circulating CD27+ memory B cells and CCR4+ T cells occurring in association with elevated EBV loads in XLP patients surviving primary EBV infection. Blood 103, 1625–1631 (2004).
Yin, L. et al. Mice deficient in the X-linked lymphoproliferative disease gene sap exhibit increased susceptibility to murine gamma-herpesvirus-68 and hypogammaglobulinemia. J. Med. Virol. 71, 446–455 (2003).
Hron, J.D., Caplan, L., Gerth, A.J., Schwartzberg, P.L. & Peng, S.L. SH2D1A regulates T-dependent humoral autoimmunity. J. Exp. Med. 200, 261–266 (2004).
Crotty, S., Kersh, E.N., Cannons, J., Schwartzberg, P.L. & Ahmed, R. SAP is required for generating long-term humoral immunity. Nature 421, 282–287 (2003).
Purtilo, D.T. et al. X-linked recessive progressive combined variable immunodeficiency (Duncan's disease). Lancet 1, 935–941 (1975).
Seemayer, T.A. et al. X-linked Lymphoproliferative Disease. in Infectious Causes of Cancer, Targets for Intervention (ed. Goedert, J.J.) 51–61 (Humana Press, Totowa, 2000).
Latour, S. et al. Binding of SAP SH2 domain to FynT SH3 domain reveals a novel mechanism of receptor signaling in immune regulation. Nat. Cell. Biol. 5, 149–154 (2003).
Gadue, P., Morton, N. & Stein, P.L. The Src family tyrosine kinase Fyn regulates natural killer T cell development. J. Exp. Med. 190, 1189–1196 (1999).
Eberl, G., Lowin-Kropf, B. & MacDonald, H.R. Cutting edge: NKT cell development is selectively impaired in Fyn-deficient mice. J. Immunol. 163, 4091–4094 (1999).
Carnaud, C. et al. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J. Immunol. 163, 4647–4650 (1999).
Chen, Y.H., Chiu, N.M., Mandal, M., Wang, N. & Wang, C.R. Impaired NK1+ T cell development and early IL-4 production in CD1-deficient mice. Immunity 6, 459–467 (1997).
Mendiratta, S.K. et al. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity 6, 469–477 (1997).
Smiley, S.T., Kaplan, M.H. & Grusby, M.J. Immunoglobulin E production in the absence of interleukin-4-secreting CD1-dependent cells. Science 275, 977–979 (1997).
Wengler, G.S. et al. A PCR-based non-radioactive X-chromosome inactivation assay for genetic counseling in X-linked primary immunodeficiencies. Life Sci. 61, 1405–1411 (1997).
Wang, N. et al. The cell surface receptor SLAM controls T cell and macrophage functions. J. Exp. Med. 199, 1255–1264 (2004).
Roberts, T.J., Lin, Y., Spence, P.M., Van Kaer, L. & Brutkiewicz, R.R. CD1d1-dependent control of the magnitude of an acute antiviral immune response. J. Immunol. 172, 3454–3461 (2004).
Smyth, M.J. et al. NKT cells - conductors of tumor immunity? Curr. Opin. Immunol. 14, 165–171 (2002).
Galli, G. et al. CD1d-restricted help to B cells by human invariant natural killer T lymphocytes. J. Exp. Med. 197, 1051–1057 (2003).
Stein, P.L., Lee, H.M., Rich, S. & Soriano, P. pp59fyn mutant mice display differential signaling in thymocytes and peripheral T cells. Cell 70, 741–750 (1992).
Pear, W.S. et al. Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood 92, 3780–3792 (1998).
Acknowledgements
We thank the patients who participated in this study. We are also grateful to R. Buckley, L. Silverman, A. Malbran, G. Uzel, S. Adelstein, R. Walls, F. Alvaro and D. Anderson for providing patient samples. We thank A. Bendelac for providing the Cd1d1−/− mice, W. Pear for the MIGR vector, M. Kronenberg and L. Sidobre for the αGC-loaded tetramer and the NKT cell hybridoma, L. Lanier for the Ly49D and Ly49G2 antibodies and Kirin Brewery for αGC. We acknowledge X. Zhong, M. Weiss, V. Shapiro and J. Stadanlick for their review of this manuscript, G. Bunin for assistance with statistics, and S. Jain for his technical assistance. This work was supported by the American Society of Hematology (K.E.N.), the Juvenile Diabetes Foundation, International (P.L.S.), the US National Institutes of Health (K.E.N., P.L.S.), the New South Wales Cancer Council (S.G.T.) and the University of Sydney, Australia (C.S.M.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Sap−/− mice have normal numbers of T and NK cells. (PDF 88 kb)
Rights and permissions
About this article
Cite this article
Nichols, K., Hom, J., Gong, SY. et al. Regulation of NKT cell development by SAP, the protein defective in XLP. Nat Med 11, 340–345 (2005). https://doi.org/10.1038/nm1189
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1189
This article is cited by
-
Thymic development of unconventional T cells: how NKT cells, MAIT cells and γδ T cells emerge
Nature Reviews Immunology (2020)
-
NK cell recognition of hematopoietic cells by SLAM-SAP families
Cellular & Molecular Immunology (2019)
-
SLAM receptors foster iNKT cell development by reducing TCR signal strength after positive selection
Nature Immunology (2019)