Abstract
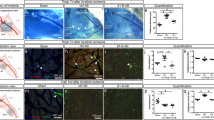
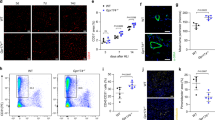
Vascular endothelial growth factor (VEGF)-induced blood vessel growth is involved in both physiological and pathological angiogenesis and requires integrin-mediated signaling. We now show that an integrin-binding protein initially described in milk-fat globule, MFG-E8 (also known as lactadherin), is expressed in and around blood vessels and has a crucial role in VEGF-dependent neovascularization in the adult mouse. Using neutralizing antibodies and lactadherin-deficient animals, we show that lactadherin interacts with αvβ3 and αvβ5 integrins and alters both VEGF-dependent Akt phosphorylation and neovascularization. In the absence of VEGF, lactadherin administration induced αvβ3- and αvβ5-dependent Akt phosphorylation in endothelial cells in vitro and strongly improved postischemic neovascularization in vivo. These results show a crucial role for lactadherin in VEGF-dependent neovascularization and identify lactadherin as an important target for the modulation of neovascularization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 9, 653–660 (2003).
Giancotti, F.G. & Ruoslahti, E. Integrin signaling. Science 285, 1028–1032 (1999).
Borges, E., Jan, Y. & Ruoslahti, E. Platelet-derived growth factor receptor beta and vascular endothelial growth factor receptor 2 bind to the beta 3 integrin through its extracellular domain. J. Biol. Chem. 275, 39867–39873 (2000).
Ho, H.K. et al. Developmental endothelial locus-1 (Del-1), a novel angiogenic protein: its role in ischemia. Circulation 109, 1314–1319 (2004).
Zhong, J. et al. Neovascularization of ischemic tissues by gene delivery of the extracellular matrix protein Del-1. J. Clin. Invest. 112, 30–41 (2003).
Hidai, C. et al. Cloning and characterization of developmental endothelial locus-1: an embryonic endothelial cell protein that binds the alphavbeta3 integrin receptor. in Genes Dev. 12, 21–33 (1998).
Stubbs, J.D. et al. cDNA cloning of a mouse mammary epithelial cell surface protein reveals the existence of epidermal growth factor-like domains linked to factor VIII-like sequences. Proc. Natl. Acad. Sci. USA 87, 8417–8421 (1990).
Taylor, M.R., Couto, J.R., Scallan, C.D., Ceriani, R.L. & Peterson, J.A. Lactadherin (formerly BA46), a membrane-associated glycoprotein expressed in human milk and breast carcinomas, promotes ArgGlyAsp (RGD)-dependent cell adhesion. DNA Cell Biol. 16, 861–869 (1997).
Andersen, M.H., Graversen, H., Fedosov, S.N., Petersen, T.E. & Rasmussen, J.T. Functional analyses of two cellular binding domains of bovine lactadherin. Biochemistry 39, 6200–6206 (2000).
Hanayama, R. et al. Identification of a factor that links apoptotic cells to phagocytes. Nature 9, 182–187 (2002)
Newburg, D.S. et al. Role of human-milk lactadherin in protection against symptomatic rotavirus infection. Lancet 351, 1160–1164 (1998).
Ensslin, M.A. & Shur, B.D. Identification of mouse sperm SED1, a bimotif EGF repeat and discoidin-domain protein involved in sperm-egg binding. Cell 114, 405–417 (2003).
Hanayama, R. et al. Autoimmune Disease and Impaired Uptake of Apoptotic Cells in MFG-E8-Deficient Mice. Science 304, 1147–1150 (2004).
Akakura, S. et al. The opsonin MFG-E8 is a ligand for the alphavbeta5 integrin and triggers DOCK180-dependent Rac1 activation for the phagocytosis of apoptotic cells. Exp. Cell Res. 292, 403–416 (2004).
Oshima, K., Aoki, N., Kato, T., Kitajima, K. & Matsuda, T. Secretion of a peripheral membrane protein, MFG-E8, as a complex with membrane vesicles. Eur. J. Biochem. 269, 1209–1218 (2002).
Miyasaka, K., Hanayama, R., Tanaka, M. & Nagata, S. Expression of milk fat globule epidermal growth factor 8 in immature dendritic cells for engulfment of apoptotic cells. Eur. J. Immunol. 34, 1414–1422 (2004).
Thery, C. et al. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J. Cell Biol. 147, 599–610 (1999).
Skarnes, W.C., Moss, J.E., Hurtley, S.M. & Beddington, R.S. Capturing genes encoding membrane and secreted proteins important for mouse development. Proc. Natl. Acad. Sci. USA 92, 6592–6596 (1995).
Mitchell, K.J. et al. Functional analysis of secreted and transmembrane proteins critical to mouse development. Nat. Genet. 28, 241–249 (2001).
Silvestre, J.S. et al. Vascular endothelial growth factor-B promotes in vivo angiogenesis. Circ. Res. 93, 114–123 (2003).
Kureishi, Y. et al. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase AKT and promotes angiogenesis in normocholesterolemic animals. Nat. Med. 6, 1004–1010 (2000).
Gerber, H.P. et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3¢-kinase/AKT signal transduction pathway. Requirement for Flk-1/KDR activation. J. Biol. Chem. 273, 30336–30343 (1998).
Hood, J.D., Frausto, R., Kiosses, W.B., Schwartz, M.A. & Cheresh, D.A. Differential alphav integrin-mediated Ras-ERK signaling during two pathways of angiogenesis. J. Cell Biol. 162, 933–943 (2003).
Oshima, K. et al. Lactation-dependent expression of an mRNA splice variant with an exon for a multiply O-glycosylated domain of mouse milk fat globule glycoprotein MFG-E8. Biochem. Biophys. Res. Commun. 254, 522–528 (1999).
Reynolds, L.E. et al. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat. Med. 8, 27–34 (2002).
Yang, H.T., Deschenes, M.R., Ogilvie, R.W. & Terjung, R.L. Basic fibroblast growth factor increases collateral blood flow in rats with femoral arterial ligation. Circ. Res. 79, 62–69 (1996).
Sullivan, C.J., Doetschman, T. & Hoying, J.B. Targeted disruption of the Fgf2 gene does not affect vascular growth in the mouse ischemic hindlimb. J. Appl. Physiol. 93, 2009–2017 (2002).
Tamarat, R., Silvestre, J.S., Durie, M. & Levy, B.I. Angiotensin II angiogenic effect in vivo involves vascular endothelial growth factor- and inflammation-related pathways. Lab. Invest. 82, 747–756 (2002).
Silvestre, J.S. et al. Regulation of matrix metalloproteinase activity in ischemic tissue by interleukin-10: role in ischemia-induced angiogenesis. Circ. Res. 89, 259–264 (2001).
Hodivala-Dilke, K.M. et al. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J. Clin. Invest. 103, 229–238 (1999).
Acknowledgements
The authors thank INSERM, Institut Curie, European Community (grant QLRT-2001-00093) and Ligue Nationale contre le Cancer for funding; H. Laurell and H. Prats (INSERM U397, Toulouse, France) for kind gift of bFGF expression plasmid; K. Mitchell and W. Skarnes (Wellcome Trust, Cambridge, UK) for providing the ES cell line (see http://baygenomics.ucsf.edu). INSERM U689 and Cardiovascular Research Institute of Mastricht are partners of the European Vascular Genomics Network (EVGN), a Network of Excellence granted by the European Commission (contract No. LSHM-CT-2003-503254) and the contribution of S. Heeneman was undertaken in the context of EVGN.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Generation of lactadherin-deficient mice. (PDF 100 kb)
Supplementary Fig. 2
Lactadherin and post-ischemic neovascularization. (PDF 143 kb)
Supplementary Fig. 3
Dose-dependent effect of lactadherin antibody administration. (PDF 110 kb)
Supplementary Fig. 4
Role of lactadherin in bFGF pro-angiogenic effect. (PDF 132 kb)
Supplementary Fig. 5
Putative model of lactadherin pro-angiogenic signaling. (PDF 96 kb)
Rights and permissions
About this article
Cite this article
Silvestre, JS., Théry, C., Hamard, G. et al. Lactadherin promotes VEGF-dependent neovascularization. Nat Med 11, 499–506 (2005). https://doi.org/10.1038/nm1233
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1233
This article is cited by
-
Proteomics of mouse brain endothelium uncovers dysregulation of vesicular transport pathways during aging
Nature Aging (2024)
-
Extracellular vesicle-derived silk fibroin nanoparticles loaded with MFGE8 accelerate skin ulcer healing by targeting the vascular endothelial cells
Journal of Nanobiotechnology (2023)
-
Milk fat-globule epidermal growth factor 8: A potential Regulator of Cutaneous Wound Healing
Molecular Biology Reports (2022)
-
Medin co-aggregates with vascular amyloid-β in Alzheimer’s disease
Nature (2022)
-
Inframe insertion and splice site variants in MFGE8 associate with protection against coronary atherosclerosis
Communications Biology (2022)