Abstract
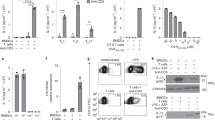
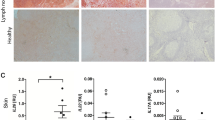
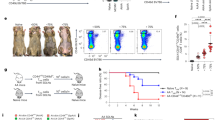
Leprosy enables investigation of mechanisms by which the innate immune system contributes to host defense against infection, because in one form, the disease progresses, and in the other, the infection is limited. We report that Toll-like receptor (TLR) activation of human monocytes induces rapid differentiation into two distinct subsets: DC-SIGN+ CD16+ macrophages and CD1b+ DC-SIGN− dendritic cells. DC-SIGN+ phagocytic macrophages were expanded by TLR-mediated upregulation of interleukin (IL)-15 and IL-15 receptor. CD1b+ dendritic cells were expanded by TLR-mediated upregulation of granulocyte-macrophage colony-stimulating factor (GM-CSF) and its receptor, promoted T cell activation and secreted proinflammatory cytokines. Whereas DC-SIGN+ macrophages were detected in lesions and after TLR activation in all leprosy patients, CD1b+ dendritic cells were not detected in lesions or after TLR activation of peripheral monocytes in individuals with the progressive lepromatous form, except during reversal reactions in which bacilli were cleared by T helper type 1 (TH1) responses. In tuberculoid lepromatous lesions, DC-SIGN+ cells were positive for macrophage markers, but negative for dendritic cell markers. Thus, TLR-induced differentiation of monocytes into either macrophages or dendritic cells seems to crucially influence effective host defenses in human infectious disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lemaitre, B., Nicolas, E., Michaut, L., Reichhart, J.M., & Hoffmann, J.A. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86, 973–983 (1996).
Takeuchi, O., Hoshino, K., & Akira, S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to staphylococcus aureus infection. J. Immunol. 165, 5392–5396 (2000).
O'Brien, A.D. et al. Genetic control of susceptibility to Salmonella typhimurium in mice: role of the LPS gene. J. Immunol. 124, 20–24 (1980).
Alexopoulou, L. et al. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in. Nat. Med. 8, 878–884 (2002).
Krutzik, S.R. et al. Activation and regulation of Toll-like receptors 2 and 1 in human leprosy. Nat. Med. 9, 525–532 (2003).
Blander, J.M. & Medzhitov, R. Regulation of phagosome maturation by signals from toll-like receptors. Science 304, 1014–1018 (2004).
Doyle, S.E. et al. Toll-like receptors induce a phagocytic gene program through p38. J. Exp. Med. 199, 81–90 (2004).
Thoma-Uszynski, S. et al. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science 291, 1544–1547 (2001).
Medzhitov, R., Preston-Hurlburt, P. & Janeway, C.A.J. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388, 394–397 (1997).
Brightbill, H.D. et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 285, 732–736 (1999).
Hertz, C.J. et al. Microbial lipopeptides stimulate dendritic cell maturation via TLR2. J. Immunol. 166, 2444–2450 (2000).
Porcelli, S.A. & Modlin, R.L. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu. Rev. Immunol. 17, 297–329 (1999).
Beckman, E.M. et al. Recognition of a lipid antigen by CD1-restricted αβ+ T cells. Nature 372, 691–694 (1994).
Sieling, P.A. et al. CD1-restricted T cell recognition of microbial lipoglycans. Science 269, 227–230 (1995).
Moody, D.B. et al. Structural requirements for glycolipid antigen recognition by CD1b-restricted T cells. Science 278, 283–286 (1997).
Engering, A. et al. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J. Immunol. 168, 2118–2126 (2002).
Geijtenbeek, T.B. et al. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100, 575–585 (2000).
Geijtenbeek, T.B. et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 197, 7–17 (2003).
Tailleux, L. et al. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J. Exp. Med. 197, 121–127 (2003).
Appelmelk, B.J. et al. Cutting edge: carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J. Immunol. 170, 1635–1639 (2003).
Cambi, A. et al. The C-type lectin DC-SIGN (CD209) is an antigen-uptake receptor for Candida albicans on dendritic cells. Eur. J. Immunol. 33, 532–538 (2003).
Colmenares, M., Puig-Kroger, A., Pello, O.M., Corbi, A.L. & Rivas, L. Dendritic cell (DC)-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin (DC-SIGN, CD209), a C-type surface lectin in human DCs, is a receptor for Leishmania amastigotes . J. Biol. Chem. 277, 36766–36769 (2002).
Wemambu, S.N.C., Turk, J.L., Waters, M.F.R. & Rees, R.J.W. Erythema nodosum leprosum: a clinical manifestation of the Arthus phenomenon. Lancet 2, 933–935 (1969).
Salgame, P. et al. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science 254, 279–282 (1991).
Yamamura, M. et al. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science 254, 277–279 (1991).
Yamamura, M. et al. Cytokine patterns of immunologically mediated tissue damage. J. Immunol. 149, 1470–1475 (1992).
Kadowaki, N. et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194, 863–869 (2001).
Graeber, T.G. & Eisenberg, D. Bioinformatic identification of potential autocrine signaling loops in cancers from gene expression profiles. Nat. Genet. 29, 295–300 (2001).
Ochoa, M.T. et al. T-cell release of granulysin contributes to host defense in leprosy. Nat. Med. 7, 174–179 (2001).
Soilleux, E.J. et al. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro . J. Leukoc. Biol 71, 445–457 (2002).
Chehimi, J. et al. HIV-1 transmission and cytokine-induced expression of DC-SIGN in human monocyte-derived macrophages. J. Leukoc. Biol. 74, 757–763 (2003).
van Lent, P.L. et al. Expression of the dendritic cell-associated C-type lectin DC-SIGN by inflammatory matrix metalloproteinase-producing macrophages in rheumatoid arthritis synovium and interaction with intercellular adhesion molecule 3-positive T cells. Arthritis Rheum. 48, 360–369 (2003).
Giordano, D., Magaletti, D.M., Clark, E.A., & Beavo, J.A. Cyclic nucleotides promote monocyte differentiation toward a DC-SIGN+ (CD209) intermediate cell and impair differentiation into dendritic cells. J. Immunol. 171, 6421–6430 (2003).
Geijtenbeek, T.B. et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100, 587–597 (2000).
Relloso, M. et al. DC-SIGN (CD209) expression is IL-4 dependent and is negatively regulated by IFN, TGF-beta, and anti-inflammatory agents. J. Immunol. 168, 2634–2643 (2002).
Judge, A.D., Zhang, X., Fujii, H., Surh, C.D. & Sprent, J. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8(+) T cells. J. Exp. Med. 196, 935–946 (2002).
Schluns, K.S., Williams, K., Ma, A., Zheng, X.X. & Lefrancois, L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J. Immunol. 168, 4827–4831 (2002).
Pulendran, B. et al. Dendritic cells generated in the presence of GM-CSF plus IL-15 prime potent CD8+ Tc1 responses in vivo . Eur. J. Immunol. 34, 66–73 (2004).
Hirschfeld, M., Ma, Y., Weis, J.H., Vogel, S.N., & Weis, J.J. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J. Immunol. 165, 618–622 (2000).
Takeuchi, O. et al. Cutting edge: Preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a toll-like receptor 2- and MyD88-dependent signaling pathway. J. Immunol. 164, 554–557 (2000).
Ridley, D.S. & Jopling, W.H. Classification of leprosy according to immunity. A five-group system. Int. J. Lepr. 34, 255–273 (1966).
Kim, J. et al. Determinants of T cell reactivity to the Mycobacterium leprae GroES homologue. J. Immunol. 159, 335–343 (1997).
Stenger, S. et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science 282, 121–125 (1998).
Acknowledgements
We would like to thank P. Brennan (Colorado State University and NIAID Contract NO1 AI-75320) for the gift of the M. leprae bacterioferritin major membrane protein II (MMPII) monoclonal antibody. We would also like to thank K. Grossheider for her technical help. This work was supported in part by grants from the US National Institutes of Health (AI07126, AI22553, AI47866).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
TLR2/1 activation triggers the modulation of an additional 5 ligand/receptor signaling pairs. (PDF 141 kb)
Supplementary Fig. 2
Activation via TLR2/1 with the tri-acylated Pam3CSK4 lipopeptide triggers monocyte differentiation in a pattern identical to the mycobacterial 19-kDa lipopeptide. (PDF 158 kb)
Supplementary Fig. 3
Peripheral monocytes from L-lep patients do not have a deficiency in responding to recombinant GM-CSF and expressing CD1b. (PDF 59 kb)
Supplementary Fig. 4
Analysis of DC-SIGN+ cells in human tonsil reveals a pattern similar to that found in T-lep lesions. (PDF 152 kb)
Supplementary Fig. 5
Toll-like receptor activation leads to the differentiation of monocytes into dendritic cells, with antigen presentation function, and macrophages, with phagocytic capacity. (PDF 113 kb)
Rights and permissions
About this article
Cite this article
Krutzik, S., Tan, B., Li, H. et al. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat Med 11, 653–660 (2005). https://doi.org/10.1038/nm1246
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1246
This article is cited by
-
CD1-mediated immune responses in mucosal tissues: molecular mechanisms underlying lipid antigen presentation system
Experimental & Molecular Medicine (2023)
-
Skin immunity: dissecting the complex biology of our body's outer barrier
Mucosal Immunology (2022)
-
Myeloid differentiation primary response protein 88 (MyD88)-deficient dendritic cells exhibit a skewed cytokine response to BCG
BMC Research Notes (2019)
-
A toll-like receptor agonist mimicking microbial signal to generate tumor-suppressive macrophages
Nature Communications (2019)
-
A T-cell receptor escape channel allows broad T-cell response to CD1b and membrane phospholipids
Nature Communications (2019)