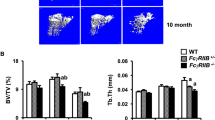
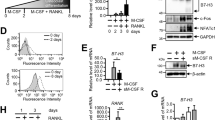
Abstract
Immunosuppressants are crucial in the prevention of detrimental immune reactions associated with allogenic organ transplantation, but they often cause adverse effects in a number of biological systems, including the skeletal system1,2. Calcineurin inhibitors FK506 and cyclosporin A inhibit nuclear factor of activated T cells (NFAT) activity and induce strong immunosuppression3,4,5. Among NFAT proteins, NFATc1 is crucial for the differentiation of bone-resorbing osteoclasts6,7. Here we show FK506 administration induces the reduction of bone mass despite a blockade of osteoclast differentiation. This reduction is caused by severe impairment of bone formation, suggesting that NFAT transcription factors also have an important role in the transcriptional program of osteoblasts. In fact, bone formation is inhibited in Nfatc1- and Nfatc2-deficient cells as well as in FK506-treated osteoblasts. Overexpression of NFATc1 stimulates Osterix8-dependent activation of the Col1a1 (encoding type I collagen) promoter, but not Runx2-dependent activation of the Bglap1 (encoding osteocalcin) promoter9. NFAT and Osterix form a complex that binds to DNA, and this interaction is important for the transcriptional activity of Osterix. Thus, NFAT and Osterix cooperatively control osteoblastic bone formation. These results may provide important insight into the management of post-transplantation osteoporosis as well as a new strategy for promoting bone regeneration in osteopenic disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rodino, M.A. & Shane, E. Osteoporosis after organ transplantation. Am. J. Med. 104, 459–469 (1998).
Leidig-Bruckner, G. et al. Frequency and predictors of osteoporotic fractures after cardiac or liver transplantation: a follow-up study. Lancet 357, 342–347 (2001).
Liu, J. et al. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 66, 807–815 (1991).
Hogan, P.G., Chen, L., Nardone, J. & Rao, A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 17, 2205–2232 (2003).
Crabtree, G.R. & Olson, E.N. NFAT signaling: choreographing the social lives of cells. Cell 109 Suppl., S67–S79 (2002).
Takayanagi, H. et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 3, 889–901 (2002).
Koga, T. et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature 428, 758–763 (2004).
Nakashima, K. et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108, 17–29 (2002).
Ducy, P., Zhang, R., Geoffroy, V., Ridall, A.L. & Karsenty, G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89, 747–754 (1997).
Karsenty, G. & Wagner, E.F. Reaching a genetic and molecular understanding of skeletal development. Dev. Cell 2, 389–406 (2002).
Cvetkovic, M. et al. The deleterious effects of long-term cyclosporine A, cyclosporine G, and FK506 on bone mineral metabolism in vivo. Transplantation 57, 1231–1237 (1994).
Fukunaga, J. et al. Expression of osteoclast differentiation factor and osteoclastogenesis inhibitory factor in rat osteoporosis induced by immunosuppressant FK506. Bone 34, 425–431 (2004).
Wang, E.A. et al. Recombinant human bone morphogenetic protein induces bone formation. Proc. Natl. Acad. Sci. USA 87, 2220–2224 (1990).
Tang, L. et al. FK506 enhanced osteoblastic differentiation in mesenchymal cells. Cell Biol. Int. 26, 75–84 (2002).
Ogawa, T. et al. Osteoblastic differentiation is enhanced by rapamycin in rat osteoblast-like osteosarcoma (ROS 17/2.8) cells. Biochem. Biophys. Res. Commun. 249, 226–230 (1998).
Murshed, M., Harmey, D., Millan, J.L., McKee, M.D. & Karsenty, G. Unique coexpression in osteoblasts of broadly expressed genes accounts for the spatial restriction of ECM mineralization to bone. Genes Dev. 19, 1093–1104 (2005).
Ranger, A.M. et al. The nuclear factor of activated T cells (NFAT) transcription factor NFATp (NFATc2) is a repressor of chondrogenesis. J. Exp. Med. 191, 9–22 (2000).
Tomita, M., Reinhold, M.I. & Molkentin, J.D. & Naski, M.C. Calcineurin and NFAT4 induce chondrogenesis. J. Biol. Chem. 277, 42214–42218 (2002).
Zhou, S., Glowacki, J. & Yates, K.E. Comparison of TGF-β/BMP pathway signaled by demineralized bone powder and BMP-2 in human dermal fibroblasts. J. Bone Miner. Res. 19, 1732–1741 (2004).
Komori, T. et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89, 755–764 (1997).
Otto, F. et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 89, 765–771 (1997).
Massague, J. How cells read TGF-β signals. Nat. Rev. Mol. Cell Biol. 1, 169–178 (2000).
de la Pompa, J.L. et al. Role of the NF-ATc transcription factor in morphogenesis of cardiac valves and septum. Nature 392, 182–186 (1998).
Ranger, A.M. et al. The transcription factor NF-ATc is essential for cardiac valve formation. Nature 392, 186–190 (1998).
Blackham, A. & Griffiths, R.J. The effect of FK506 and cyclosporin A on antigen-induced arthritis. Clin. Exp. Immunol. 86, 224–228 (1991).
Yocum, D.E. et al. Efficacy and safety of tacrolimus in patients with rheumatoid arthritis: a double-blind trial. Arthritis Rheum. 48, 3328–3337 (2003).
Takayanagi, H. Mechanistic insight into osteoclast differentiation in osteoimmunology. J. Mol. Med. 83, 170–179 (2005).
Kim, S. et al. Stat1 functions as a cytoplasmic attenuator of Runx2 in the transcriptional program of osteoblast differentiation. Genes Dev. 17, 1979–1991 (2003).
Hodge, M.R. et al. Hyperproliferation and dysregulation of IL-4 expression in NF-ATp-deficient mice. Immunity 4, 397–405 (1996).
Takayanagi, H. et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-γ. Nature 408, 600–605 (2000).
Acknowledgements
We are grateful to T. W. Mak and L. Glimcher for providing Nfatc1−/− and Nfatc2−/− mice. We thank G. Karsenty, K. Imamura, M.A. Brown, RNAi Co., Ltd., and Fujisawa Pharmaceutical Co., Ltd. for providing plasmids and reagents. We also thank M. Noda, I. Morita, A. Izumi, T. Kohro, H. Murayama, T. Hongo, K. Sato, M. Shinohara, K. Nishikawa, H.J. Göber, S. Harumiya, A. Suematsu, Y. Kim, S. Ochi, M. Isobe, J. Hirooka, N. Kumasaki and I. Kawai for discussion and technical assistance. This work was supported in part by PRESTO and SORST programs of JST, grants for Genome Network Project from Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT), grants for the 21st century COE program, Grants-in-Aid for Scientific Research from Japan Society for the Promotion of Science and MEXT, Health Sciences Research Grants from the Ministry of Health, Labour and Welfare of Japan, grants from the Mitsubishi Foundation, the Kato Trust for Nambyo Research, Takeda Science Foundation, Daiwa Securities Health Foundation, the Naito Foundation, Kowa Life Science Foundation, Suzuken Memorial Foundation, Kato Memorial Bioscience Foundation, Inamori Foundation, Uehara Memorial Foundation and the Nakajima Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Koga, T., Matsui, Y., Asagiri, M. et al. NFAT and Osterix cooperatively regulate bone formation. Nat Med 11, 880–885 (2005). https://doi.org/10.1038/nm1270
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1270
This article is cited by
-
SP7: from Bone Development to Skeletal Disease
Current Osteoporosis Reports (2023)
-
ATP transporters in the joints
Purinergic Signalling (2021)
-
miR-143 promotes angiogenesis and osteoblast differentiation by targeting HDAC7
Cell Death & Disease (2020)
-
Effect of immunosuppressants on a mouse model of osteogenesis imperfecta type V harboring a heterozygous Ifitm5 c.-14C > T mutation
Scientific Reports (2020)
-
NFATc2-rearranged sarcomas: clinicopathologic, molecular, and cytogenetic study of 7 cases with evidence of AGGRECAN as a novel diagnostic marker
Modern Pathology (2020)