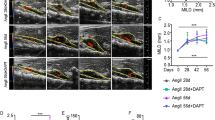
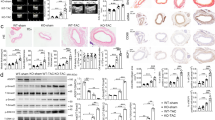
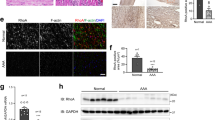
Abstract
Abdominal aortic aneurysm (AAA) is a common disease among elderly people that, when surgical treatment is inapplicable, results in progressive expansion and rupture of the aorta with high mortality. Although nonsurgical treatment for AAA is much awaited, few options are available because its molecular pathogenesis remains elusive. Here, we identify JNK as a proximal signaling molecule in the pathogenesis of AAA. Human AAA tissue showed a high level of phosphorylated JNK. We show that JNK programs a gene expression pattern in different cell types that cooperatively enhances the degradation of the extracellular matrix while suppressing biosynthetic enzymes of the extracellular matrix. Selective inhibition of JNK in vivo not only prevented the development of AAA but also caused regression of established AAA in two mouse models. Thus, JNK promotes abnormal extracellular matrix metabolism in the tissue of AAA and may represent a therapeutic target.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Curci, J.A., Lee, J.K. & Thompson, R.W. Pathogenesis of Abdominal Aortic Aneurysm. in Current Therapy in Vascular Surgery (eds. Ernst, C.B. & Stanley, J.C.) 199–206 (Elsevier, Philadelphia, 2001).
Upchurch, G.R., Jr. Gene therapy to treat aortic aneurysms: right goal, wrong strategy. Circulation 112, 939–940 (2005).
Pyo, R. et al. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J. Clin. Invest. 105, 1641–1649 (2000).
Longo, G.M. et al. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J. Clin. Invest. 110, 625–632 (2002).
Allaire, E., Forough, R., Clowes, M., Starcher, B. & Clowes, A.W. Local overexpression of TIMP-1 prevents aortic aneurysm degeneration and rupture in a rat model. J. Clin. Invest. 102, 1413–1420 (1998).
Krettek, A., Sukhova, G.K. & Libby, P. Elastogenesis in human arterial disease: a role for macrophages in disordered elastin synthesis. Arterioscler. Thromb. Vasc. Biol. 23, 582–587 (2003).
Huffman, M.D. et al. Functional importance of connective tissue repair during the development of experimental abdominal aortic aneurysms. Surgery 128, 429–438 (2000).
Rowe, D., McGoodwin, E., Martin, G. & Grahn, D. Decreased lysyl oxidase activity in the aneurysm-prone, mottled mouse. J. Biol. Chem. 252, 939–942 (1977).
Bode, M.K. et al. Increased amount of type III pN-collagen in AAA when compared with AOD. Eur. J. Vasc. Endovasc. Surg. 23, 413–420 (2002).
Bigatel, D.A. et al. The matrix metalloproteinase inhibitor BB-94 limits expansion of experimental abdominal aortic aneurysms. J. Vasc. Surg. 29, 130–138 (1999).
Petrinec, D. et al. Doxycycline inhibition of aneurysmal degeneration in an elastase-induced rat model of abdominal aortic aneurysm: preservation of aortic elastin associated with suppressed production of 92 kD gelatinase. J. Vasc. Surg. 23, 336–346 (1996).
Prall, A.K. et al. Doxycycline in patients with abdominal aortic aneurysms and in mice: comparison of serum levels and effect on aneurysm growth in mice. J. Vasc. Surg. 35, 923–929 (2002).
Mosorin, M. et al. Use of doxycycline to decrease the growth rate of abdominal aortic aneurysms: a randomized, double-blind, placebo-controlled pilot study. J. Vasc. Surg. 34, 606–610 (2001).
Baxter, B.T. et al. Prolonged administration of doxycycline in patients with small asymptomatic abdominal aortic aneurysms: report of a prospective (Phase II) multicenter study. J. Vasc. Surg. 36, 1–12 (2002).
Allaire, E. et al. Vascular smooth muscle cell endovascular therapy stabilizes already developed aneurysms in a model of aortic injury elicited by inflammation and proteolysis. Ann. Surg. 239, 417–427 (2004).
Buth, J. & Harris, P. Endovascular Treatment of Aortic Aneurysms. in Vascular Surgery (ed. Rutherford, R.B.) 1452–1475 (Elsevier, Philadelphia, 2005).
Patel, M.I., Melrose, J., Ghosh, P. & Appleberg, M. Increased synthesis of matrix metalloproteinases by aortic smooth muscle cells is implicated in the etiopathogenesis of abdominal aortic aneurysms. J. Vasc. Surg. 24, 82–92 (1996).
Manning, A.M. & Davis, R.J. Targeting JNK for therapeutic benefit: from junk to gold? Nat. Rev. Drug Discov. 2, 554–565 (2003).
Galis, Z.S. & Khatri, J.J. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ. Res. 90, 251–262 (2002).
Mietus-Snyder, M., Glass, C.K. & Pitas, R.E. Transcriptional activation of scavenger receptor expression in human smooth muscle cells requires AP-1/c-Jun and C/EBPbeta. Arterioscler. Thromb. Vasc. Biol. 18, 1440–1449 (1998).
Yan, L., Borregaard, N., Kjeldsen, L. & Moses, M.A. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J. Biol. Chem. 276, 37258–37265 (2001).
Maki, J. et al. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation 106, 2503–2509 (2002).
Ricci, R. et al. Requirement of JNK2 for scavenger receptor A-mediated foam cell formation in atherogenesis. Science 306, 1558–1561 (2004).
Bagowski, C.P. & Ferrell, J.E., Jr. Bistability in the JNK cascade. Curr. Biol. 11, 1176–1182 (2001).
Borsello, T. et al. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat. Med. 9, 1180–1186 (2003).
Daugherty, A., Manning, M.W. & Cassis, L.A. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J. Clin. Invest. 105, 1605–1612 (2000).
Shin, M. et al. An inhibitor of c-jun aminoterminal kinase (SP600125) represses c-Jun activation, DNA-binding and PMA-inducible 92-kDa type IV collagenase expression. Biochim. Biophys. Acta 1589, 311–316 (2002).
Gum, R., Wang, H., Lengyel, E., Juarez, J. & Boyd, D. Regulation of 92 kDa type IV collagenase expression by the jun aminoterminal kinase- and the extracellular signal-regulated kinase-dependent signaling cascades. Oncogene 14, 1481–1493 (1997).
Cho, A., Graves, J. & Reidy, M.A. Mitogen-activated protein kinases mediate matrix metalloproteinase-9 expression in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 20, 2527–2532 (2000).
Ventura, J.J., Kennedy, N.J., Flavell, R.A. & Davis, R.J. JNK regulates autocrine expression of TGF-beta1. Mol. Cell 15, 269–278 (2004).
Leask, A., Holmes, A., Black, C.M. & Abraham, D.J. Connective tissue growth factor gene regulation. Requirements for its induction by transforming growth factor-beta 2 in fibroblasts. J. Biol. Chem. 278, 13008–13015 (2003).
Schlumberger, W., Thie, M., Rauterberg, J. & Robenek, H. Collagen synthesis in cultured aortic smooth muscle cells. Modulation by collagen lattice culture, transforming growth factor-beta 1, and epidermal growth factor. Arterioscler. Thromb. 11, 1660–1666 (1991).
Dai, J. et al. Overexpression of transforming growth factor-beta1 stabilizes already-formed aortic aneurysms: a first approach to induction of functional healing by endovascular gene therapy. Circulation 112, 1008–1015 (2005).
Takagi, Y., Ishikawa, M., Nozaki, K., Yoshimura, S. & Hashimoto, N. Increased expression of phosphorylated c-Jun amino-terminal kinase and phosphorylated c-Jun in human cerebral aneurysms. Neurosurgery 51, 997–1002 (2002).
Parodi, F.E., Mao, D., Ennis, T.L., Bartoli, M.A. & Thompson, R.W. Suppression of experimental abdominal aortic aneurysms in mice by treatment with pyrrolidine dithiocarbamate, an antioxidant inhibitor of nuclear factor-kappaB. J. Vasc. Surg. 41, 479–489 (2005).
Nakashima, H. et al. Inhibition of experimental abdominal aortic aneurysm in the rat by use of decoy oligodeoxynucleotides suppressing activity of nuclear factor kappaB and ets transcription factors. Circulation 109, 132–138 (2004).
Kim, H.S., Luo, L., Pflugfelder, S.C. & Li, D.Q. Doxycycline inhibits TGF-beta1-induced MMP-9 via Smad and MAPK pathways in human corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 46, 840–848 (2005).
Sato, H. & Seiki, M. Regulatory mechanism of 92 kDa type IV collagenase gene expression which is associated with invasiveness of tumor cells. Oncogene 8, 395–405 (1993).
Papa, S., Zazzeroni, F., Pham, C.G., Bubici, C. & Franzoso, G. Linking JNK signaling to NF-kappaB: a key to survival. J. Cell Sci. 117, 5197–5208 (2004).
Bennett, B.L. et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. USA 98, 13681–13686 (2001).
Adachi, M. et al. Proteasome-dependent decrease in Akt by growth factors in vascular smooth muscle cells. FEBS Lett. 554, 77–80 (2003).
Aoki, H. et al. Direct activation of mitochondrial apoptosis machinery by c-Jun N-terminal kinase in adult cardiac myocytes. J. Biol. Chem. 277, 10244–10250 (2002).
Zempo, N. et al. Matrix metalloproteinases of vascular wall cells are increased in balloon-injured rat carotid artery. J. Vasc. Surg. 20, 209–217 (1994).
Palamakumbura, A.H. & Trackman, P.C. A fluorometric assay for detection of lysyl oxidase enzyme activity in biological samples. Anal. Biochem. 300, 245–251 (2002).
Acknowledgements
We thank D. Boyle and G.S. Firestein for suggestions, S. Saito, M. Oishi and T. Hozawa for technical assistance and E.O. Weinberg, H. Suzuki and H. Oda for critical reading. This work was supported in part by Grant-in-aid for Scientific Research (KAKENHI 12770651, 14657284 and 17591337 (to K.Y.), 12670673, 12204081, 14370229 and 16390365 (to H.A.), 12770344 (to K.F.) and 16209026 (to M.M.)) from MEXT Japan, Japan Heart Foundation/Zeria Pharmaceutical Grant for Research on Cardiovascular Disease (to H.A.), New Frontier Project from Yamaguchi University (to H.A. and K.Y.) and a Grant from Sankyo Company for the Department of Molecular Cardiovascular Biology, Yamaguchi University School of Medicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Expression and effect of JNK activity modifiers in VSMCs. (PDF 185 kb)
Supplementary Fig. 2
Definition of JNK Dependence Index. (PDF 170 kb)
Supplementary Fig. 3
Phosphorylated JNK in control, AAA and AOD tissues. (PDF 200 kb)
Supplementary Fig. 4
The role of JNK in expression and secretion of MMPs. (PDF 236 kb)
Supplementary Fig. 5
Determination of aortic internal diameter in live mice by ultrasonography. (PDF 293 kb)
Supplementary Table
The entire list of JNK-regulated genes. (PDF 479 kb)
Rights and permissions
About this article
Cite this article
Yoshimura, K., Aoki, H., Ikeda, Y. et al. Regression of abdominal aortic aneurysm by inhibition of c-Jun N-terminal kinase. Nat Med 11, 1330–1338 (2005). https://doi.org/10.1038/nm1335
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1335
This article is cited by
-
The mechanism and therapy of aortic aneurysms
Signal Transduction and Targeted Therapy (2023)
-
Aortic pathology from protein kinase G activation is prevented by an antioxidant vitamin B12 analog
Nature Communications (2019)
-
Ginkgo biloba extracts prevent aortic rupture in angiotensin II-infused hypercholesterolemic mice
Acta Pharmacologica Sinica (2019)
-
The potential of cardiac rehabilitation as a method of suppressing abdominal aortic aneurysm expansion: a pilot study
Heart and Vessels (2019)
-
Abdominal aortic aneurysms
Nature Reviews Disease Primers (2018)