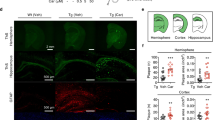
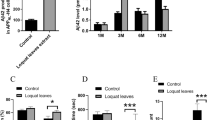
Abstract
When given orally to a transgenic mouse model of Alzheimer disease, cyclohexanehexol stereoisomers inhibit aggregation of amyloid β peptide (Aβ) into high-molecular-weight oligomers in the brain and ameliorate several Alzheimer disease–like phenotypes in these mice, including impaired cognition, altered synaptic physiology, cerebral Aβ pathology and accelerated mortality. These therapeutic effects, which occur regardless of whether the compounds are given before or well after the onset of the Alzheimer disease–like phenotype, support the idea that the accumulation of Aβ oligomers has a central role in the pathogenesis of Alzheimer disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Notes
NOTE: In the version of this article initially published online, there was an error in Table 1. In the 6-month prophylactic study under Scyllo-cyclohexanehexol, the soluble Aβ40 level should be 105 ± 8 instead of 1,105 ± 8. The error has been corrected for the HTML and print versions of the article.
References
Selkoe, D.J. Deciphering the genesis and fate of amyloid beta-protein yields novel therapies for Alzheimer disease. J. Clin. Invest. 110, 1375–1381 (2002).
Glabe, C.C. Amyloid accumulation and pathogenesis of Alzheimer's disease: significance of monomeric, oligomeric and fibrillar Aβ. Subcell. Biochem. 38, 167–177 (2005).
McLaurin, J. & Chakrabartty, A. Membrane disruption by Alzheimer beta-amyloid peptides mediated through specific binding to either phospholipids or gangliosides. Implications for neurotoxicity. J. Biol. Chem. 271, 26482–26489 (1996).
McLaurin, J. & Chakrabartty, A. Characterization of the interactions of Alzheimer beta-amyloid peptides with phospholipid membranes. Eur. J. Biochem. 245, 355–363 (1997).
Koppaka, V. & Axelson, P.H. Accelerated accumulation of amyloid beta proteins on oxidatively damaged lipid membranes. Biochemistry 39, 10011–10016 (2000).
Mizuno, T. et al. Cholesterol-dependent generation of a seeding amyloid beta-protein in cell culture. J. Biol. Chem. 274, 15110–15114 (1999).
Choo-Smith, L.P. & Surewicz, W.K. The interaction between Alzheimer amyloid beta(1–40) peptide and ganglioside GM1-containing membranes. FEBS Lett. 402, 95–98 (1997).
Yanagisawa, K. et al. GM1 ganglioside-bound amyloid beta-protein (A beta): a possible form of preamyloid in Alzheimer's disease. Nat. Med. 1, 1062–1066 (1995).
Auton, M. & Bolen, D.W. Predicting the energetics of osmolyte-induced protein folding/unfolding. Proc. Natl. Acad. Sci. USA 102, 15065–15068 (2005).
McLaurin, J., Franklin, T., Chakrabartty, A. & Fraser, P.E. Phosphatidylinositol and inositol involvement in Alzheimer amyloid-beta fibril growth and arrest. J. Mol. Biol. 278, 183–194 (1998).
McLaurin, J., Goloumb, R., Jurewicz, A., Antel, J.P. & Fraser, P.E. Inositol stereoisomers stabilize an oligomeric aggregate of Alzheimer amyloid beta peptide and inhibit Aβ-induced toxicity. J. Biol. Chem. 275, 18495–18502 (2000).
Chishti, M.A. et al. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J. Biol. Chem. 276, 21562–21570 (2001).
Janus, C. et al. Aβ peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature 408, 979–982 (2000).
Morris, R. Development of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 11, 47–60 (1984).
Klein, W.L. Aβ toxicity in Alzheimer's disease: globular oligomers (ADDLs) as a new vaccine and drug targets. Neurochem. Int. 41, 345–352 (2002).
Kayed, R. et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300, 486–489 (2003).
Spector, R. Myo-inositol transport through the blood-brain barrier. Neurochem. Res. 13, 785–787 (1988).
Uldry, M. et al. Identification of a mammalian H(+)-myo-inositol symporter expressed predominantly in the brain. EMBO J. 20, 4467–4477 (2001).
Uldry, M. & Thorens, B. The SLC2 family of facilitated hexose and polyol transporters. Pflugers Arch. 447, 480–489 (2004).
Shetty, H.U. & Hollway, H.W. Assay of myo-inositol in cerebrospinal fluid and plasma by chemical ionization mass spectrometry of the hexaacetate derivative. Biol. Mass Spectrom. 23, 440–444 (1994).
McLaurin, J. et al. Therapeutically effective antibodies against amyloid-beta peptide target amyloid-beta residues 4–10 and inhibit cytotoxicity and fibrillogenesis. Nat. Med. 8, 1263–1269 (2002).
Frenkel, D., Katz, O. & Solomon, B. Immunization against Alzheimer's β-amyloid plaques via EFRH phage administration. Proc. Natl. Acad. Sci. USA 97, 11455–11459 (2000).
Bard, F. et al. Epitope and isotype specificities of antibodies to β-amyloid peptide for protection against disease-like neuropathology. Proc. Natl. Acad. Sci. USA 100, 2023–2028 (2003).
Nicoll, J.A. et al. Neuropathology of human Alzheimer disease after immunization with amyloid-β peptide: a case report. Nat. Med. 9, 448–452 (2003).
Hock, C. et al. Antibodies against beta-amyloid slow cognitive decline in Alzheimer's disease. Neuron 38, 547–554 (2003).
Tanaka, M. et al. Trehalose alleviates polyglutamine-mediated pathology in a mouse model of Huntington disease. Nat. Med. 10, 148–154 (2004).
Lesne, S. et al. A specific amyloid-β protein assembly in the brain impairs memory. Nature 440, 352–357 (2006).
Fisher, S.K.,, Novak, J.E., & Agranoff, B.W. Inositol and higher inositol phosphates in neural tissues: homeostasis, metabolism and functional significance. J. Neurochem. 82, 736–754 (2002).
Vaucher, E. et al. Object recognition memory and cholinergic parameters in mice expressing human presenilin 1 transgenes. Exp. Neurol. 175, 398–406 (2002).
Mount, H.T. et al. Progressive sensorimotor impairment is not associated with reduced dopamine and high energy phosphate donors in a model of ataxia-telangiectasia. J. Neurochem. 88, 1449–1454 (2004).
Wiltfang, J. et al. Highly conserved and disease-specific patterns of carboxyterminally truncated Abeta peptides 1–37/38/39 in addition to 1–40/42 in Alzheimer's disease and in patients with chronic neuroinflammation. J. Neurochem. 81, 481–496 (2002).
Haccou, P. & Mellis, E. Statistical Analysis of Behavioural Data 120–186 (Oxford Univ Press, Oxford, 1995).
Chen, F. et al. Carboxyl-terminal fragments of Alzheimer beta-amyloid precursor protein accumulate in restricted and unpredicted intracellular compartments in presenilin 1-deficient cells. J. Biol. Chem. 275, 36794–36802 (2000).
Phinney, A. et al. No hippocampal neuron or synaptic bouton loss in learning-impaired aged β-amyloid precursor protein-null mice. Neuroscience 90, 1207–1216 (1999).
Hu, L. et al. The impact of Aβ-plaques on cortical cholinergic and non-cholinergic presynaptic boutons in Alzheimer's disease-like transgenic mice. Neuroscience 121, 421–432 (2003).
Acknowledgements
The authors thank C. Glabe for the oligomeric specific antibody, P. Mathews for the C1/6.1 antibody and R. Ahrens and P. Horne for technical assistance. The authors acknowledge support from the Ontario Alzheimer's Society (to H.T.J.M., P.H., P.E.F., D.W., J.M.), Canadian Institutes of Health Research (to H.T.J.M., P.H., P.E.F., D.W., J.M.), the Natural Sciences and Engineering Research Council of Canada (to J.M.), Canadian Foundation for Innovation (to H.T.J.M., P.H., P.E.F., D.W., J.M.), Ontario Research and Development Challenge Fund (to J.M., P.H., D.W., P.E.F.) Howard Hughes Medical Institute (to P.H.) and the Scottish Rite and Cryptic Foundations (to J.M.).
Author information
Authors and Affiliations
Contributions
J.M., A.L.P., H.T.J.M. and P.S.G.-H. designed research. M.E.K., M.E.B., C.A.H., M.H.L.L., A.A.D., J.E.C., J.E.F., M.F.L., F.C. and S.S.N.W. performed research. J.M., M.E.K., C.A.H., A.L.P. and H.T.J.M. analyzed the data. J.M., H.T.J.M., P.E.F., D.W. and P.S.G.-H. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
JoAnne McLaurin, Paul E. Fraser, David Westaway and Peter St. George-Hyslop and the University of Toronto have licensed this technology to a private-sector company from which support for a research program is derived.
JoAnne McLaurin is named as inventor on patent applications relating to this technology.
Supplementary information
Supplementary Fig. 1
Cyclohexanehexol stereoisomer structures. (PDF 16 kb)
Supplementary Fig. 2
Spatial reference memory version of the Morris water maze test in 6-month-old TgCRND8 mice untreated and treated with mannitol. (PDF 98 kb)
Supplementary Fig. 3
At 6 months of age, the plaque burden and astrogliosis in TgCRND8 mice untreated, epi- and scyllo-cyclohexanehexoltreated mice were examined. (PDF 240 kb)
Supplementary Fig. 4
A cue test was performed at the end of the spatial memory version of the Morris water maze test. (PDF 25 kb)
Supplementary Fig. 5
In vitro γ-secretase assay in HEK293 cells trasfected with human PPswe. (PDF 143 kb)
Supplementary Table 1
Overall effect of cyclohexanehexols on cognitive function. (PDF 26 kb)
Rights and permissions
About this article
Cite this article
McLaurin, J., Kierstead, M., Brown, M. et al. Cyclohexanehexol inhibitors of Aβ aggregation prevent and reverse Alzheimer phenotype in a mouse model. Nat Med 12, 801–808 (2006). https://doi.org/10.1038/nm1423
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1423
This article is cited by
-
Constitutive glucose dehydrogenase elevates intracellular NADPH levels and luciferase luminescence in Bacillus subtilis
Microbial Cell Factories (2022)
-
Therapeutic roles of plants for 15 hypothesised causal bases of Alzheimer’s disease
Natural Products and Bioprospecting (2022)
-
Engineering Bacillus subtilis Cells as Factories: Enzyme Secretion and Value-added Chemical Production
Biotechnology and Bioprocess Engineering (2020)
-
A new-generation of Bacillus subtilis cell factory for further elevated scyllo-inositol production
Microbial Cell Factories (2017)
-
A guanidine-appended scyllo-inositol derivative AAD-66 enhances brain delivery and ameliorates Alzheimer’s phenotypes
Scientific Reports (2017)