Abstract
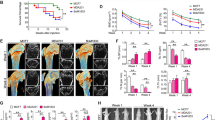
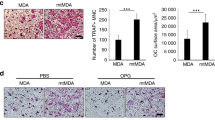
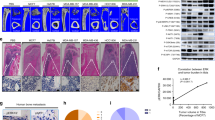
Advanced breast cancers frequently metastasize to bone, resulting in osteolytic lesions, yet the underlying mechanisms are poorly understood. Here we report that nuclear factor–κB (NF-κB) plays a crucial role in the osteolytic bone metastasis of breast cancer by stimulating osteoclastogenesis. Using an in vivo bone metastasis model, we found that constitutive NF-κB activity in breast cancer cells is crucial for the bone resorption characteristic of osteolytic bone metastasis. We identified the gene encoding granulocyte macrophage–colony stimulating factor (GM-CSF) as a key target of NF-κB and found that it mediates osteolytic bone metastasis of breast cancer by stimulating osteoclast development. Moreover, we observed that the expression of GM-CSF correlated with NF-κB activation in bone-metastatic tumor tissues from individuals with breast cancer. These results uncover a new and specific role of NF-κB in osteolytic bone metastasis through GM-CSF induction, suggesting that NF-κB is a potential target for the treatment of breast cancer and the prevention of skeletal metastasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Roodman, G.D. Mechanisms of bone metastasis. N. Engl. J. Med. 350, 1655–1664 (2004).
Mundy, G.R. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2, 584–593 (2002).
Javed, A. et al. Impaired intranuclear trafficking of Runx2 (AML3/CBFA1) transcription factors in breast cancer cells inhibits osteolysis in vivo. Proc. Natl. Acad. Sci. USA 102, 1454–1459 (2005).
Kozlow, W. & Guise, T.A. Breast cancer metastasis to bone: mechanisms of osteolysis and implications for therapy. J. Mammary Gland Biol. Neoplasia 10, 169–180 (2005).
Zhang, J. et al. Osteoprotegerin inhibits prostate cancer–induced osteoclastogenesis and prevents prostate tumor growth in the bone. J. Clin. Invest. 107, 1235–1244 (2001).
Martin, T.J. Paracrine regulation of osteoclast formation and activity: milestones in discovery. J. Musculoskelet. Neuronal Interact. 4, 243–253 (2004).
Fidler, I.J. Critical determinants of metastasis. Semin. Cancer Biol. 12, 89–96 (2002).
Chambers, A.F., Groom, A.C. & MacDonald, I.C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2, 563–572 (2002).
Huang, S., Pettaway, C.A., Uehara, H., Bucana, C.D. & Fidler, I.J. Blockade of NF-κB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene 20, 4188–4197 (2001).
Huber, M.A. et al. NF-κB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J. Clin. Invest. 114, 569–581 (2004).
Kang, Y. et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3, 537–549 (2003).
Guise, T.A. et al. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J. Clin. Invest. 98, 1544–1549 (1996).
Yin, J.J. et al. TGF-β signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J. Clin. Invest. 103, 197–206 (1999).
Sovak, M.A. et al. Aberrant nuclear factor-κB/Rel expression and the pathogenesis of breast cancer. J. Clin. Invest. 100, 2952–2960 (1997).
Hu, M.C. et al. IκB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell 117, 225–237 (2004).
Eddy, S.F. et al. Inducible IκB kinase/IκB kinase ε expression is induced by CK2 and promotes aberrant nuclear factor-κB activation in breast cancer cells. Cancer Res. 65, 11375–11383 (2005).
Cailleau, R., Young, R., Olive, M. & Reeves, W.J., Jr. Breast tumor cell lines from pleural effusions. J. Natl. Cancer Inst. 53, 661–674 (1974).
Wang, C.-Y., Mayo, M.W. & Baldwin, A.S. TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science 274, 784–787 (1996).
Wang, C.-Y., Mayo, M.W., Korneluk, R.C., Goeddel, D.V. & Baldwin, A.S. NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281, 1680–1683 (1998).
Wang, C.-Y., Cusack, J., Liu, R. & Baldwin, A.S. Control of inducible chemoresistance: enhanced anti-tumor therapy via increased apoptosis through inhibition of NF-κB. Nat. Med. 5, 412–417 (1999).
Baxter, A. et al. Hit-to-lead studies: the discovery of potent, orally active, thiophenecarboxamide IKK-2 inhibitors. Bioorg. Med. Chem. Lett. 14, 2817–2822 (2004).
Mayo, M.W. & Baldwin, A.S. The transcription factor NF-κB: control of oncogenesis and cancer therapy resistance. Biochim. Biophys. Acta 1470, M55–M62 (2000).
Greten, F.R. et al. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118, 285–296 (2004).
Dranoff, G. Cytokines in cancer pathogenesis and cancer therapy. Nat. Rev. Cancer 4, 11–22 (2004).
Yoneda, T. & Hiraga, T. Crosstalk between cancer cells and bone microenvironment in bone metastasis. Biochem. BioPhys. Res. Commun. 328, 679–687 (2005).
Jones, D.H. et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature 440, 692–696 (2006).
Hamilton, J.A. GM-CSF in inflammation and autoimmunity. Trends Immunol. 23, 403–408 (2002).
Hodge, J.M. et al. Osteoclastic potential of human CFU-GM: biophasic effect of GM-CSF. J. Bone Miner. Res. 19, 190–199 (2004).
MacDonald, B.R. et al. Effects of human recombinant CSF-GM and highly purified CSF-1 on the formation of multinucleated cells with osteoclast characteristics in long-term bone marrow cultures. J. Bone Miner. Res. 1, 227–233 (1986).
Menaa, C. et al. Annexin II increases osteoclast formation by stimulating the proliferation of osteoclast precursors in human marrow cultures. J. Clin. Invest. 103, 1605–1613 (1999).
Myint, Y.Y. et al. Granulocyte/macrophage colony-stimulating factor and interleukin-3 correct osteopetrosis in mice with osteopetrosis mutation. Am. J. Pathol. 154, 553–566 (1999).
Li, F. et al. Annexin II stimulates RANKL expression through MAPK. J. Bone Miner. Res. 20, 1161–1167 (2005).
Coussens, L.M. & Werb, Z. Inflammation and cancer. Nature 420, 860–867 (2002).
Hanahan, D. & Weinberg, R.A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Clark, E.A., Golub, T.R., Lander, E.S. & Hynes, R.O. Genomic analysis of metastasis reveals an essential role for RhoC. Nature 406, 532–535 (2000).
Horak, C.E. & Steeg, P.S. Metastasis gets site specific. Cancer Cell 8, 93–95 (2005).
Jimi, E. et al. Selective inhibition of NF-κB blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat. Med. 10, 617–624 (2004).
Ruocco, M.G. et al. IκB kinase (IKK)β, but not IKKα, is a critical mediator of osteoclast survival and is required for inflammation-induced bone loss. J. Exp. Med. 201, 1677–1687 (2005).
Morgan, H., Tumber, A. & Hill, P.A. Breast cancer cells induce osteoclast formation by stimulating host IL-11 production and downregulating granulocyte/macrophage colony-stimulating factor. Int. J. Cancer 109, 653–660 (2004).
Luo, J.L., Maeda, S., Hsu, L.C., Yagita, H. & Karin, M. Inhibition of NF-κB in cancer cells converts inflammation-induced tumor growth mediated by TNFα to TRAIL-mediated tumor regression. Cancer Cell 6, 297–305 (2004).
Queen, M.M., Ryan, R.E., Holzer, R.G., Keller-Peck, C.R. & Jorcyk, C.L. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Res. 65, 8896–8904 (2005).
Lin, A. & Karin, M. NF-κB in cancer: a marked target. Semin. Cancer Biol. 13, 107–114 (2003).
Condeelis, J. & Pollard, J.W. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 124, 263–266 (2006).
Li, Q. & Verma, I.M. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2, 725–734 (2002).
Chen, Z.J. Ubiquitin signalling in the NF-κB pathway. Nat. Cell Biol. 7, 758–765 (2005).
Zeng, Q. et al. Crosstalk between tumor and endothelial cells promotes angiogenesis through MAPK activation of Notch. Cancer Cell 8, 13–23 (2005).
Acknowledgements
We thank A. Baldwin and D. Guttridge for discussion, T. Guise (University of Virginia) for providing breast cancer cells, and E. Tang, D. Saims, A. Rehman and R. Franceschi for their comments on the manuscript. This study was supported by the US National Institutes of Health (grants DE015618, CA100849 and CA093900).
Author information
Authors and Affiliations
Contributions
B.K.P., S.C. and C.-Y.W. designed and organized the experiments, performed the animal studies, analyzed the data; generated the figures and wrote the manuscript. H.Z. performed the subcloning. Q.Z., T.G., K.G., V.S., L.P. and R.J.Z. conducted the histological analysis. J.D., S.S., E.T.K. and L.M. performed the animal studies and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Constitutive NF-κB in breast cancer cells inhibits tumor growth. (PDF 252 kb)
Supplementary Fig. 2
The IKK2 inhibitor VI suppresses subcutaneous tumor growth in vivo. MDA-MB-231 cells (1 × 106) were injected into nude mice subcutaneously and treated with the IKK2 inhibitor VI for 3 weeks, then tumor sizes were measured. (PDF 86 kb)
Supplementary Fig. 3
NF-κB promotes cell invasion in vitro. (PDF 135 kb)
Supplementary Fig. 4
GM-CSF does not activate tumor cells through an antocrine mechanism. (PDF 214 kb)
Supplementary Fig. 5
GM-CSF is highly expressed in bone-metastatic breast tumors from human patients. (PDF 316 kb)
Supplementary Fig. 6
The inhibition of GM-CSF function did not affect tumor cell proliferation. (PDF 43 kb)
Supplementary Fig. 7
GM-CSF-specific antibodies inhibit breast cancer metastasis to bone. (PDF 187 kb)
Rights and permissions
About this article
Cite this article
Park, B., Zhang, H., Zeng, Q. et al. NF-κB in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSF. Nat Med 13, 62–69 (2007). https://doi.org/10.1038/nm1519
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1519
This article is cited by
-
Transcriptomic Analysis of the Rat Dorsal Root Ganglion After Fracture
Molecular Neurobiology (2024)
-
Induction of filopodia formation by α-Actinin-2 via RelA with a feedforward activation loop promoting overt bone marrow metastasis of gastric cancer
Journal of Translational Medicine (2023)
-
Effect of tumor-associated macrophages on the pyroptosis of breast cancer tumor cells
Cell Communication and Signaling (2023)
-
The ERα/KDM6B regulatory axis modulates osteogenic differentiation in human mesenchymal stem cells
Bone Research (2022)
-
Activation of cannabinoid receptors in breast cancer cells improves osteoblast viability in cancer-bone interaction model while reducing breast cancer cell survival and migration
Scientific Reports (2022)