Abstract
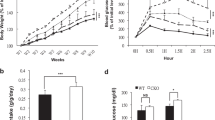
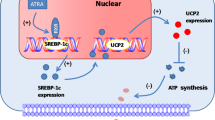
The metabolism of vitamin A and the diverse effects of its metabolites are tightly controlled by distinct retinoid-generating enzymes, retinoid-binding proteins and retinoid-activated nuclear receptors. Retinoic acid regulates differentiation and metabolism by activating the retinoic acid receptor and retinoid X receptor (RXR), indirectly influencing RXR heterodimeric partners. Retinoic acid is formed solely from retinaldehyde (Rald), which in turn is derived from vitamin A. Rald currently has no defined biologic role outside the eye. Here we show that Rald is present in rodent fat, binds retinol-binding proteins (CRBP1, RBP4), inhibits adipogenesis and suppresses peroxisome proliferator-activated receptor-γ and RXR responses. In vivo, mice lacking the Rald-catabolizing enzyme retinaldehyde dehydrogenase 1 (Raldh1) resisted diet-induced obesity and insulin resistance and showed increased energy dissipation. In ob/ob mice, administrating Rald or a Raldh inhibitor reduced fat and increased insulin sensitivity. These results identify Rald as a distinct transcriptional regulator of the metabolic responses to a high-fat diet.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Paydas, S. et al. Vasculitis associated with all trans retinoic acid (ATRA) in a case with acute promyelocytic leukemia. Leuk. Lymphoma 44, 547–548 (2003).
Redlich, C.A. et al. Effect of long-term β-carotene and vitamin A on serum cholesterol and triglyceride levels among participants in the Carotene and Retinol Efficacy Trial (CARET). Atherosclerosis 143, 427–434 (1999).
Rapola, J.M. et al. Randomised trial of α-tocopherol and β-carotene supplements on incidence of major coronary events in men with previous myocardial infarction. Lancet 349, 1715–1720 (1997).
Napoli, J.L. Retinoic acid: its biosynthesis and metabolism. Prog. Nucleic Acid Res. Mol. Biol. 63, 139–188 (1999).
Duester, G., Mic, F.A. & Molotkov, A. Cytosolic retinoid dehydrogenases govern ubiquitous metabolism of retinol to retinaldehyde followed by tissue-specific metabolism to retinoic acid. Chem. Biol. Interact. 143–144, 201–210 (2003).
Chambon, P. A decade of molecular biology of retinoic acid receptors. FASEB J. 10, 940–954 (1996).
Heyman, R.A. et al. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell 68, 397–406 (1992).
Shulman, A.I. & Mangelsdorf, D.J. Retinoid x receptor heterodimers in the metabolic syndrome. N. Engl. J. Med. 353, 604–615 (2005).
Fu, M. et al. A nuclear receptor atlas: 3T3–L1 adipogenesis. Mol. Endocrinol. 19, 2437–2450 (2005).
Xue, J.C., Schwarz, E.J., Chawla, A. & Lazar, M.A. Distinct stages in adipogenesis revealed by retinoid inhibition of differentiation after induction of PPARγ. Mol. Cell. Biol. 16, 1567–1575 (1996).
Kikonyogo, A., Abriola, D.P., Dryjanski, M. & Pietruszko, R. Mechanism of inhibition of aldehyde dehydrogenase by citral, a retinoid antagonist. Eur. J. Biochem. 262, 704–712 (1999).
Ress, N.B. et al. Toxicology and carcinogenesis studies of microencapsulated citral in rats and mice. Toxicol. Sci. 71, 198–206 (2003).
Duester, G. Families of retinoid dehydrogenases regulating vitamin A function: production of visual pigment and retinoic acid. Eur. J. Biochem. 267, 4315–4324 (2000).
Molotkov, A. & Duester, G. Genetic evidence that retinaldehyde dehydrogenase Raldh1 (Aldh1a1) functions downstream of alcohol dehydrogenase Adh1 in metabolism of retinol to retinoic acid. J. Biol. Chem. 278, 36085–36090 (2003).
von Lintig, J. & Wyss, A. Molecular analysis of vitamin A formation: cloning and characterization of β-carotene 15,15′-dioxygenases. Arch. Biochem. Biophys. 385, 47–52 (2001).
Lakshman, M.R., Mychkovsky, I. & Attlesey, M. Enzymatic conversion of all-trans-β-carotene to retinal by a cytosolic enzyme from rabbit and rat intestinal mucosa. Proc. Natl. Acad. Sci. USA 86, 9124–9128 (1989).
Vogel, S. et al. Characterization of a new member of the fatty acid-binding protein family that binds all-trans-retinol. J. Biol. Chem. 276, 1353–1360 (2001).
Berni, R., Clerici, M., Malpeli, G., Cleris, L. & Formelli, F. Retinoids: in vitro interaction with retinol-binding protein and influence on plasma retinol. FASEB J. 7, 1179–1184 (1993).
Yu, S. et al. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor γ1 (PPARγ1) overexpression. J. Biol. Chem. 278, 498–505 (2003).
Iwaki, M. et al. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes 52, 1655–1663 (2003).
Repa, J.J., Hanson, K.K. & Clagett-Dame, M. All-trans-retinol is a ligand for the retinoic acid receptors. Proc. Natl. Acad. Sci. USA 90, 7293–7297 (1993).
Berger, J.P. et al. Distinct properties and advantages of a novel peroxisome proliferator-activated protein γ selective modulator. Mol. Endocrinol. 17, 662–676 (2003).
Canan Koch, S.S. et al. Synthesis of retinoid X receptor-specific ligands that are potent inducers of adipogenesis in 3T3–L1 cells. J. Med. Chem. 42, 742–750 (1999).
Imai, T., Jiang, M., Chambon, P. & Metzger, D. Impaired adipogenesis and lipolysis in the mouse upon selective ablation of the retinoid X receptor α mediated by a tamoxifen-inducible chimeric Cre recombinase (Cre-ERT2) in adipocytes. Proc. Natl. Acad. Sci. USA 98, 224–228 (2001).
Green, H. & Meuth, M. An established pre-adipose cell line and its differentiation in culture. Cell 3, 127–133 (1974).
Puigserver, P. et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839 (1998).
He, W. et al. Adipose-specific peroxisome proliferator-activated receptor γ knockout causes insulin resistance in fat and liver but not in muscle. Proc. Natl. Acad. Sci. USA 100, 15712–15717 (2003).
Kubota, N. et al. Pioglitazone ameliorates insulin resistance and diabetes by both adiponectin dependent and independent pathway. J. Biol. Chem. 281, 8748–8755 (2006).
Yang, Q. et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 436, 356–362 (2005).
Lee, S., Bacha, F., Gungor, N. & Arslanian, S.A. Racial differences in adiponectin in youth: relationship to visceral fat and insulin sensitivity. Diabetes Care 29, 51–56 (2006).
Nawrocki, A.R. et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor γ agonists. J. Biol. Chem. 281, 2654–2660 (2006).
Maeda, K. et al. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab. 1, 107–119 (2005).
Graham, T.E. et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N. Engl. J. Med. 354, 2552–2563 (2006).
Hallsten, K. et al. Rosiglitazone but not metformin enhances insulin- and exercise-stimulated skeletal muscle glucose uptake in patients with newly diagnosed type 2 diabetes. Diabetes 51, 3479–3485 (2002).
Yamauchi, T. et al. Inhibition of RXR and PPARγ ameliorates diet-induced obesity and type 2 diabetes. J. Clin. Invest. 108, 1001–1013 (2001).
Cavasotto, C.N. et al. Determinants of retinoid X receptor transcriptional antagonism. J. Med. Chem. 47, 4360–4372 (2004).
Sell, H. et al. Peroxisome proliferator-activated receptor γ agonism increases the capacity for sympathetically mediated thermogenesis in lean and ob/ob mice. Endocrinology 145, 3925–3934 (2004).
Fliers, E. et al. White adipose tissue: getting nervous. J. Neuroendocrinol. 15, 1005–1010 (2003).
Ziouzenkova, O. et al. Lipolysis of triglyceride-rich lipoproteins generates PPAR ligands: evidence for an antiinflammatory role for lipoprotein lipase. Proc. Natl. Acad. Sci. USA 100, 2730–2735 (2003).
Xie, Y., Lashuel, H.A., Miroy, G.J., Dikler, S. & Kelly, J.W. Recombinant human retinol-binding protein refolding, native disulfide formation, and characterization. Protein Expr. Purif. 14, 31–37 (1998).
Cogan, U., Kopelman, M., Mokady, S. & Shinitzky, M. Binding affinities of retinol and related compounds to retinol binding proteins. Eur. J. Biochem. 65, 71–78 (1976).
Weir, J.B. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. (Lond.) 109, 1–9 (1949).
Szatmari, I. et al. PPARγ controls CD1d expression by turning on retinoic acid synthesis in developing human dendritic cells. J. Exp. Med. 203, 2351–2362 (2006).
Acknowledgements
We thank G. Sukhova (Brigham and Women's Hospital), J. Kirkland and T. Tchkonia (Boston University), N. Krinsky and R. Russell (Tufts University) for helpful discussions; P. Scherer, S. Kliewer, D. Mangelsdorf (University of Texas), C.H. Lee (Harvard University) and T. Willson for reagents; and E. Shvarz, K. Volz, J. Qin, T. Archibald, N. Sharma, S. Laclair and R. Driscoll for technical support. This research was supported by the Boston Obesity Nutrition Research Center 5P30DK046200 and K12 HD051959-01 NICHD BIRCWH, the American Heart Association SDG 0530101N (O.Z.); the US National Institutes of Health (R01 HL071745 and P01 HL48743) and the Donald W. Reynolds Foundation (J.P.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
The metabolism of vitamin A generates Rald and retinoic acid. (PDF 56 kb)
Supplementary Fig. 2
Chromatograms of Rald oxime extracts from lean and obese mice as compared with vitamin A and Rald standards. (PDF 119 kb)
Supplementary Fig. 3
Rald inhibits PPAR-γ–regulated adipogenic genes. (PDF 62 kb)
Supplementary Fig. 4
Additional metabolic characteristics of Raldh1−/− and wild-type mice after high-fat diet feeding. (PDF 49 kb)
Rights and permissions
About this article
Cite this article
Ziouzenkova, O., Orasanu, G., Sharlach, M. et al. Retinaldehyde represses adipogenesis and diet-induced obesity. Nat Med 13, 695–702 (2007). https://doi.org/10.1038/nm1587
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1587
This article is cited by
-
A causal relationship between antioxidants, minerals and vitamins and metabolic syndrome traits: a Mendelian randomization study
Diabetology & Metabolic Syndrome (2023)
-
Effects of high NaHCO3 alkalinity on growth, tissue structure, digestive enzyme activity, and gut microflora of grass carp juvenile
Environmental Science and Pollution Research (2023)
-
In vitro and in silico cholinesterase inhibitory potential of metabolites from Laurencia snackeyi (Weber-van Bosse) M. Masuda
3 Biotech (2023)
-
Reconnoitring the Usage of Agroindustrial Waste in Carotenoid Production for Food Fortification: a Sustainable Approach to Tackle Vitamin A Deficiency
Food and Bioprocess Technology (2023)
-
High fat diet–induced hyperlipidemia and tissue steatosis in rabbits through modulating ileal microbiota
Applied Microbiology and Biotechnology (2022)