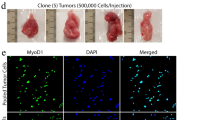
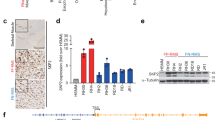
Abstract
Rhabdomyosarcoma (RMS) is the most common soft-tissue sarcoma in children, yet molecular events associated with the genesis and progression of this potentially fatal disease are largely unknown. For the molecules and pathways that have been implicated, genetic validation has been impeded by lack of a mouse model of RMS. Here we show that simultaneous loss of Ink4a/Arf function and disruption of c-Met signaling in Ink4a/Arf−/− mice transgenic for hepatocyte growth factor/scatter factor (HGF/SF) induces RMS with extremely high penetrance and short latency. In cultured myoblasts, c-Met activation and Ink4a/Arf loss suppress myogenesis in an additive fashion. Our data indicate that human c-MET and INK4a/ARF, situated at the nexus of pathways regulating myogenic growth and differentiation, represent critical targets in RMS pathogenesis. The marked synergism in mice between aberrant c-Met signaling and Ink4a/Arf inactivation, lesions individually implicated in human RMS, suggests a therapeutic combination to combat this devastating childhood cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Notes
Note: In the original version of this article published online 7 October 2002, the center lanes of each gel in Figure 4b were marked positive (+). They should have been marked negative (−). The authors regret this error. This has been corrected in the HTML version of the article and a corrigendum has been appended to the PDF version of this article. A print corrigendum will appear in a forthcoming issue of the journal.
References
Jeffers, M., Rong, S. & Vande Woude, G.F. Hepatocyte growth factor/scatter factor-Met signaling in tumorigenicity and invasion/metastasis. J. Mol. Med. 74, 505–513 (1996).
Matsumoto, K. & Nakamura, T. Emerging multipotent aspects of hepatocyte growth factor. J. Biochem. 119, 591–600 (1996).
Trusolino, L. & Comoglio, P.M. Scatter-factor and semaphorin receptors: Cell signaling for invasive growth. Nature Rev. 2, 289–300 (2002).
Danilkovitch-Miagkova, A. & Zbar, B. Dysregulation of Met receptor tyrosine kinase activity in invasive tumors. J. Clin. Invest. 109, 863–867 (2002).
Sakata, H. et al. Hepatocyte growth factor/scatter factor overexpression induces growth, abnormal development, and tumor formation in transgenic mouse livers. Cell Growth Diff. 7, 1513–1523 (1996).
Takayama, H. et al. Diverse tumorigenesis associated with aberrant development in mice overexpressing hepatocyte growth factor/scatter factor. Proc. Natl. Acad. Sci. USA 94, 701–706 (1997).
Otsuka, T. et al. c-Met autocrine activation induces development of malignant melanoma and acquisition of the metastatic phenotype. Cancer Res. 58, 5157–5167 (1998).
Foulkes, W.D., Flanders, T.Y., Pollock, P.M. & Hayward, N.K. The CDKN2A (p16) gene and human cancer. Mol. Med. 3, 5–20 (1997).
Ruas, M. & Peters, G. The p16INK4a/CDKN2A tumor suppressor and its relatives. Biochim. Biophys. Acta 1378, F115–F177 (1998).
Quelle, D.E., Zindy, F., Ashmun, R.A. & Sherr, C.J. Alternative reading frames of the INK4a tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell 83, 993–1000 (1995).
Chin, L., Pomerantz, J. & DePinho, R.A. The INK4a/ARF tumor suppressor: one gene—two products—two pathways. Trends Biochem. Sci. 23, 291–296 (1998).
Sherr, C.J. The INK4a/ARF network in tumour suppression. Nature Rev. Mol. Cell Biol. 2, 731–737 (2001).
Serrano, M. et al. Role of the INK4a locus in tumor suppression and cell mortality. Cell 85, 27–37 (1996).
Takayama, H., LaRochelle, W.J., Anver, M., Bockman, D.E. & Merlino, G. Scatter factor/hepatoctye growth factor as a regulator of skeletal muscle and neural crest development. Proc. Natl. Acad. Sci. USA 93, 5866–5871 (1996).
Merlino, G. & Helman, L.J. Rhabdomyosarcoma—working out the pathways. Oncogene 18, 5340–5348 (1999).
Ferracini, R. et al. Retrogenic expression of the MET proto-oncogene correlates with the invasive phenotype of human rhabdomyosarcomas. Oncogene 12, 1697–1705 (1996).
Ginsberg, J.P., Davis, R.J., Bennicelli, J.L., Nauta, L.E. & Barr, F.G. Up-regulation of MET but not neural cell adhesion molecule expression by the PAX3-FKHR fusion protein in alveolar rhabdomyosarcoma. Cancer Res. 58, 3542–3546 (1998).
Seale, P. et al. Pax7 is required for the specification of myogenic satellite cells. Cell 102, 777–786 (2000).
Maelandsmo, G.M. et al. Homozygous deletion frequency and expression levels of the CDKN2 gene in human sarcomas-relationship to amplification and mRNA levels of CDK4 and CCND1. Br. J. Cancer 72, 393–398 (1995).
Gu, W. et al. Interaction of myogenic factors and the retinoblastoma protein mediates muscle cell commitment and differentiation. Cell 72, 309–324 (1993).
Skapek, S.X., Rhee, J., Spicer, D.B. & Lassar, A.B. Inhibition of myogenic differentiation in proliferating myoblasts by cyclin D1-dependent kinase. Science 267, 1022–1024 (1995).
Novitch, B.G., Mulligan, G.J., Jacks, T. & Lassar, A.B. Skeletal muscle cells lacking the retinoblastoma protein display defects in muscle gene expression and accumulate in S and G2 phases of the cell cycle. J. Cell Biol. 135, 441–456 (1996).
Zacksenhaus. et al. pRb controls proliferation, differentiation, and death of skeletal muscle cells and other lineages during embryogenesis. Genes Dev. 10, 3051–3064 (1996).
Novitch, B.G., Spicer, D.B., Kim, P.S., Cheung, W.L. & Lassar, A.B. pRb is required for MEF2-dependent gene expression as well as cell-cycle arrest during skeletal muscle differentiation. Curr. Biol. 9, 449–459 (1999).
Bladt, F., Riethmacher, D., Isenmann, S., Aguzzi, A. & Birchmeier, C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature 376, 768–771 (1995).
Allen, R.E., Sheehan, S.M., Taylor, R.G., Kendall, T.L. & Rice, G.M. Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J. Cell. Physiol. 165, 307–312 (1995).
Anastasi, S. et al. A natural hepatocyte growth factor/scatter factor autocrine loop in myoblast cells and the effect of the constitutive Met kinase activation on myogenic differentiation. J. Cell Biol. 137, 1057–1068 (1997).
Tatsumi, R., Anderson, J.E., Nevoret, C.J., Halevy, O. & Allen, R.E. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev. Biol. 194, 114–128 (1998).
Gal-Levi, R., Leshem, Y., Aoki, S., Nakamura, T. & Halevy, O. Hepatocyte growth factor plays a dual role in regulating skeletal muscle satellite cell proliferation and differentiation. Biochim. Biophys. Acta 1402, 39–51 (1998).
Filmus, J. et al. Induction of cyclin D1 overexpression by activated ras. Oncogene 9, 3627–3633 (1994).
Bennett, A.M. & Tonks, N.K. Regulation of distinct stages of skeletal muscle differentiation by mitogen-activated protein kinases. Science 278, 1288–1291 (1997).
Rao, S.S., Chu, C. & Kohtz, D.S. Ectopic expression of cyclin D1 prevents activation of gene transcription by myogenic basic helix-loop-helix regulators. Mol. Cell Biol. 14, 5259–5267 (1994).
Spicer, D.B., Rhee, J., Cheung, W.L. & Lassar, A.B. Inhibition of myogenic bHLH and MEF2 transcription factors by the bHLH protein Twist. Science 272, 1476–1480 (1996).
Maestro, R. et al. Twist is a potential oncogene that inhibits apoptosis. Genes Dev. 13, 2207–2217 (1999).
Leshem, Y., Spicer, D.B., Gal-Levi, R. & Halevy, O. Hepatocyte growth factor (HGF) inhibits skeletal muscle cell differentiation: a role for the bHLH protein twist and the cdk inhibitor p27. J. Cell. Physiol. 184, 101–109 (2000).
Boccaccio, C. et al. Induction of epithelial tubules by growth factor HGF depends on the STAT pathway. Nature 391, 285–288 (1998).
Pomerantz, J. et al. The Ink4a tumor suppressor gene product, p19Arf, interacts with MDM2 and neutralizes MDM2's inhibition of p53. Cell 92, 713–723 (1998).
Allen, R.E., Temm-Grove, C.J., Sheehan, S.M. & Rice, G. Skeletal muscle satellite cell cultures. Methods Cell Biol. 52, 155–176 (1997).
Chakravarthy, M.V., Davis, B.S. & Booth, F.W. IGF-I restores satellite cell proliferative potential in immobilized old skeletal muscle. J. Appl. Physiol. 89, 1365–1379 (2000).
Acknowledgements
We thank D. Spicer, H. Takayama, G. Celli, K. Miura, P. Meltzer, L. Wakefield, G.Vande Woude and J. Ward for useful discussions; M. Tsokos for assistance with initial histopathological evaluation; J. Owens for electron microscopy; H. Wimbrow for animal colony husbandry; and B. Kasprzak and D. Butcher for immunohistochemistry. We thank the following for plasmid DNAs: G. Bell (for mouse IGF-II); B. Vogelstein (for human MDM2); Ed Harlow (for human CDK4); K. Huppi (for mouse GAPDH); E.Olson (for mouse myoD and myogenin); P. Seale (for pax7); and C. Ponzetto (for mouse myf5). This work was supported, in part, by the Cooperative Human Tissue Network, which is funded by the National Cancer Institute, and in part by NIH U01CA84313-04. R.A.D. is an American Cancer Society Research Professor.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Sharp, R., Recio, J., Jhappan, C. et al. Synergism between INK4a/ARF inactivation and aberrant HGF/SF signaling in rhabdomyosarcomagenesis. Nat Med 8, 1276–1280 (2002). https://doi.org/10.1038/nm787
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm787
This article is cited by
-
Rhabdomyosarcoma
Nature Reviews Disease Primers (2019)
-
A novel mouse model of rhabdomyosarcoma underscores the dichotomy of MDM2-ALT1 function in vivo
Oncogene (2018)
-
Exosomes derived from embryonal and alveolar rhabdomyosarcoma carry differential miRNA cargo and promote invasion of recipient fibroblasts
Scientific Reports (2016)
-
Probing for a deeper understanding of rhabdomyosarcoma: insights from complementary model systems
Nature Reviews Cancer (2015)
-
Proof-of-concept rare cancers in drug development: the case for rhabdomyosarcoma
Oncogene (2014)