Abstract
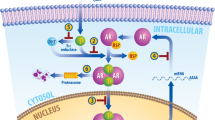
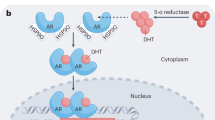
Using microarray-based profiling of isogenic prostate cancer xenograft models, we found that a modest increase in androgen receptor mRNA was the only change consistently associated with the development of resistance to antiandrogen therapy. This increase in androgen receptor mRNA and protein was both necessary and sufficient to convert prostate cancer growth from a hormone-sensitive to a hormone-refractory stage, and was dependent on a functional ligand-binding domain. Androgen receptor antagonists showed agonistic activity in cells with increased androgen receptor levels; this antagonist-agonist conversion was associated with alterations in the recruitment of coactivators and corepressors to the promoters of androgen receptor target genes. Increased levels of androgen receptor confer resistance to antiandrogens by amplifying signal output from low levels of residual ligand, and by altering the normal response to antagonists. These findings provide insight toward the design of new antiandrogens.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Feldman, B.J. & Feldman, D. The development of androgen-independent prostate cancer. Nat. Rev. Cancer 1, 34–45 (2001).
Gelmann, E.P. Molecular biology of the androgen receptor. J. Clin. Oncol. 20, 3001–3015 (2002).
Balk, S.P. Androgen receptor as a target in androgen-independent prostate cancer. Urology 60, 132–138 (2002).
Taplin, M.E. et al. Selection for androgen receptor mutations in prostate cancers treated with androgen antagonist. Cancer Res. 59, 2511–2515 (1999).
Taplin, M.E. et al. Androgen receptor mutations in androgen-independent prostate cancer: Cancer and Leukemia Group B Study 9663. J. Clin. Oncol. 21, 2673–2678 (2003).
Visakorpi, T. et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat. Genet. 9, 401–406 (1995).
Taplin, M.E. et al. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N. Engl. J. Med. 332, 1393–1398 (1995).
Veldscholte, J. et al. A mutation in the ligand binding domain of the androgen receptor of human LNCaP cells affects steroid binding characteristics and response to anti-androgens. Biochem. Biophys. Res. Commun. 173, 534–540 (1990).
Matias, P.M. et al. Structural basis for the glucocorticoid response in a mutant human androgen receptor (AR(ccr)) derived from an androgen-independent prostate cancer. J. Med. Chem. 45, 1439–1446 (2002).
Craft, N., Shostak, Y., Carey, M. & Sawyers, C.L. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat. Med. 5, 280–285 (1999).
Gioeli, D. et al. Androgen receptor phosphorylation. Regulation and identification of the phosphorylation sites. J. Biol. Chem. 277, 29304–29314 (2002).
Kato, S. et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science 270, 1491–1494 (1995).
Font de Mora, J. & Brown, M. AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol. Cell. Biol. 20, 5041–5047 (2000).
Tremblay, A., Tremblay, G.B., Labrie, F. & Giguere, V. Ligand-independent recruitment of SRC-1 to estrogen receptor β through phosphorylation of activation function AF-1. Mol. Cell 3, 513–519 (1999).
Gregory, C.W. et al. A mechanism for androgen receptor-mediated prostate cancer recurrence after androgen deprivation therapy. Cancer Res. 61, 4315–4319 (2001).
Li, P. et al. Heterogeneous expression and functions of androgen receptor co-factors in primary prostate cancer. Am. J. Pathol. 161, 1467–1474 (2002).
Glass, C.K. & Rosenfeld, M.G. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 14, 121–141 (2000).
Raffo, A.J. et al. Overexpression of bcl-2 protects prostate cancer cells from apoptosis in vitro and confers resistance to androgen depletion in vivo. Cancer Res. 55, 4438–4445 (1995).
McDonnell, T.J. et al. Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res. 52, 6940–6944 (1992).
Kinoshita, H. et al. Methylation of the androgen receptor minimal promoter silences transcription in human prostate cancer. Cancer Res. 60, 3623–3630 (2000).
Shang, Y., Myers, M. & Brown, M. Formation of the androgen receptor transcription complex. Mol. Cell 9, 601–610 (2002).
Zhau, H.Y. et al. Androgen-repressed phenotype in human prostate cancer. Proc. Natl. Acad. Sci. USA 93, 15152–15157 (1996).
Wainstein, M.A. et al. CWR22: androgen-dependent xenograft model derived from a primary human prostatic carcinoma. Cancer Res. 54, 6049–6052 (1994).
Ellis, W.J. et al. Characterization of a novel androgen-sensitive, prostate-specific antigen-producing prostatic carcinoma xenograft: LuCaP 23. Clin. Cancer Res. 2, 1039–1048 (1996).
Horoszewicz, J.S. et al. LNCaP model of human prostatic carcinoma. Cancer Res. 43, 1809–1818 (1983).
Klein, K.A. et al. Progression of metastatic human prostate cancer to androgen independence in immunodeficient SCID mice. Nat. Med. 3, 402–408 (1997).
Perou, C.M. et al. Molecular portraits of human breast tumours. Nature 406, 747–752 (2000).
Gregory, C.W., Johnson, R.T., Jr., Mohler, J.L., French, F.S. & Wilson, E.M. Androgen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgen. Cancer Res. 61, 2892–2898 (2001).
Huang, Z.Q., Li, J. & Wong, J. AR possesses an intrinsic hormone-independent transcriptional activity. Mol. Endocrinol. 16, 924–937 (2002).
Matias, P.M. et al. Structural evidence for ligand specificity in the binding domain of the human androgen receptor. Implications for pathogenic gene mutations. J. Biol. Chem. 275, 26164–26171 (2000).
Lobaccaro, J.M. et al. Molecular modeling and in vitro investigations of the human androgen receptor DNA-binding domain: application for the study of two mutations. Mol. Cell. Endocrinol. 116, 137–147 (1996).
Migliaccio, A. et al. Steroid-induced androgen receptor-oestradiol receptor β-Src complex triggers prostate cancer cell proliferation. EMBO J. 19, 5406–5417 (2000).
Kousteni, S. et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell 104, 719–730 (2001).
Manolagas, S.C., Kousteni, S. & Jilka, R.L. Sex steroids and bone. Recent Prog. Horm. Res. 57, 385–409 (2002).
DePrimo, S.E. et al. Transcriptional programs activated by exposure of human prostate cancer cells to androgen. Genome Biol. 3, RESEARCH0032 (2002).
Masiello, D., Cheng, S., Bubley, G.J., Lu, M.L. & Balk, S.P. Bicalutamide functions as an androgen receptor antagonist by assembly of a transcriptionally inactive receptor. J. Biol. Chem. 277, 26321–26326 (2002).
Edwards, J., Krishna, N.S., Grigor, K.M. & Bartlett, J.M. Androgen receptor gene amplification and protein expression in hormone refractory prostate cancer. Br. J. Cancer 89, 552–556 (2003).
Laitinen, S., Karhu, R., Sawyers, C.L., Vessella, R.L. & Visakorpi, T. Chromosomal aberrations in prostate cancer xenografts detected by comparative genomic hybridization. Genes Chromosomes Cancer 35, 66–73 (2002).
Grad, J.M., Dai, J.L., Wu, S. & Burnstein, K.L. Multiple androgen response elements and a Myc consensus site in the androgen receptor (AR) coding region are involved in androgen-mediated up-regulation of AR messenger RNA. Mol. Endocrinol. 13, 1896–1911 (1999).
Craft, N. et al. Evidence for clonal outgrowth of androgen-independent prostate cancer cells from androgen-dependent tumors through a two-step process. Cancer Res. 59, 5030–5036 (1999).
Ellwood-Yen, K. et al. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell 4, 223–238 (2003).
Wang, S. et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell 4, 209–221 (2003).
Shiau, A.K. et al. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell 95, 927–937 (1998).
Norris, J.D. et al. Peptide antagonists of the human estrogen receptor. Science 285, 744–746 (1999).
Baek, S.H. et al. Exchange of N-CoR corepressor and Tip60 coactivator complexes links gene expression by NF-κB and β-amyloid precursor protein. Cell 110, 55–67 (2002).
Shang, Y. & Brown, M. Molecular determinants for the tissue specificity of SERMs. Science 295, 2465–2468 (2002).
Schellhammer, P.F. et al. Prostate specific antigen decreases after withdrawal of antiandrogen therapy with bicalutamide or flutamide in patients receiving combined androgen blockade. J. Urol. 157, 1731–1735 (1997).
Sack, J.S. et al. Crystallographic structures of the ligand-binding domains of the androgen receptor and its T877A mutant complexed with the natural agonist dihydrotestosterone. Proc. Natl. Acad. Sci. USA 98, 4904–4909 (2001).
Zhou, Z.X., Sar, M., Simental, J.A., Lane, M.V. & Wilson, E.M. A ligand-dependent bipartite nuclear targeting signal in the human androgen receptor. Requirement for the DNA-binding domain and modulation by NH2-terminal and carboxyl-terminal sequences. J. Biol. Chem. 269, 13115–13123 (1994).
Acknowledgements
We thank C. Gregory from University of North Carolina–Chapel Hill for providing CWR22 RNA and protein; I. Mellinghoff for reagents; and K. Ellwood-Yen, M. Carey, T. Graber, J. Nicoll, J. Xu, A. Kwon and members of the Sawyers lab for helpful discussions. This work was supported by grants from the National Cancer Institute, Department of Defense and CapCURE. C.L.S. is an Investigator of the Howard Hughes Medical Institute and a Doris Duke Distinguished Clinical Scientist.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, C., Welsbie, D., Tran, C. et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med 10, 33–39 (2004). https://doi.org/10.1038/nm972
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm972
This article is cited by
-
Acquired copy number variation in prostate tumours: a review of common somatic copy number alterations, how they are formed and their clinical utility
British Journal of Cancer (2024)
-
Ubiquitination Process Mediates Prostate Cancer Development and Metastasis through Multiple Mechanisms
Cell Biochemistry and Biophysics (2024)
-
Bipolar androgen therapy plus nivolumab for patients with metastatic castration-resistant prostate cancer: the COMBAT phase II trial
Nature Communications (2024)
-
The heterogeneity and clonal evolution analysis of the advanced prostate cancer with castration resistance
Journal of Translational Medicine (2023)
-
Investigation of enzalutamide, docetaxel, and cabazitaxel resistance in the castration resistant prostate cancer cell line C4 using genome-wide CRISPR/Cas9 screening
Scientific Reports (2023)