Abstract
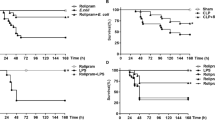
Sepsis represents a major cause of death in intensive care units. Here we show that administration of lysophosphatidylcholine (LPC), an endogenous lysophospholipid, protected mice against lethality after cecal ligation and puncture (CLP) or intraperitoneal injection of Escherichia coli. In vivo treatment with LPC markedly enhanced clearance of intraperitoneal bacteria and blocked CLP-induced deactivation of neutrophils. In vitro, LPC increased bactericidal activity of neutrophils, but not macrophages, by enhancing H2O2 production in neutrophils that ingested E. coli. Incubation with an antibody to the LPC receptor, G2A, inhibited LPC-induced protection from CLP lethality and inhibited the effects of LPC in neutrophils. G2A-specific antibody also blocked the inhibitory effects of LPC on certain actions of lipopolysaccharides (LPS), including lethality and the release of tumor necrosis factor-α (TNF-α) from neutrophils. These results suggest that LPC can effectively prevent and treat sepsis and microbial infections.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hoyert, D.L., Kochanek, K.D. & Murphy, S.L. Deaths: final data for 1997. Natl. Vital Stat. Rep. 47, 1–104 (1999).
Huber-Lang, M.S. et al. Complement-induced impairment of innate immunity during sepsis. J. Immunol. 169, 3223–3231 (2002).
Czermak, B.J. et al. Protective effects of C5a blockade in sepsis. Nat. Med. 5, 788–792 (1999).
Docke, W.D. et al. Monocyte deactivation in septic patients: restoration by IFN-γ treatment. Nat. Med. 3, 678–681 (1997).
Wang, S.D., Huang, K.J., Lin, Y.S. & Lei, H.Y. Sepsis-induced apoptosis of the thymocytes in mice. J. Immunol. 152, 5014–5021 (1994).
Ayala, A. et al. Increased mucosal B-lymphocyte apoptosis during polymicrobial sepsis is a Fas ligand but not an endotoxin-mediated process. Blood 91, 1362–1372 (1998).
Hotchkiss, R.S. et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J. Immunol. 166, 6952–6963 (2001).
Hotchkiss, R.S. & Karl, I.E. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 348, 138–150 (2003).
Bone, R.C. Sir Isaac Newton, sepsis, SIRS, and CARS. Crit. Care Med. 24, 1125–1128 (1996).
Huber-Lang, M.S. et al. Protective effects of anti-C5a peptide antibodies in experimental sepsis. FASEB J. 15, 568–570 (2001).
Hotchkiss, R.S. et al. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc. Natl. Acad. Sci. USA 96, 14541–14546 (1999).
Kabarowski, J.H., Xu, Y. & Witte, O.N. Lysophosphatidylcholine as a ligand for immunoregulation. Biochem. Pharmacol. 64, 161–167 (2002).
Quinn, M.T., Parthasarathy, S. & Steinberg, D. Lysophosphatidylcholine: a chemotactic factor for human monocytes and its potential role in atherogenesis. Proc. Natl. Acad. Sci. USA 85, 2805–2809 (1988).
Nakano, T., Raines, E.W., Abraham, J.A., Klagsbrun, M. & Ross, R. Lysophosphatidylcholine upregulates the level of heparin-binding epidermal growth factor-like growth factor mRNA in human monocytes. Proc. Natl. Acad. Sci. USA 91, 1069–1073 (1994).
Liu-Wu, Y., Hurt-Camejo, E. & Wiklund, O. Lysophosphatidylcholine induces the production of IL-1β by human monocytes. Atherosclerosis 137, 351–357 (1998).
Coutant, F. et al. Mature dendritic cell generation promoted by lysophosphatidylcholine. J. Immunol. 169, 1688–1695 (2002).
Sakai, M. et al. Lysophosphatidylcholine plays an essential role in the mitogenic effect of oxidized low density lipoprotein on murine macrophages. J. Biol. Chem. 269, 31430–31435 (1994).
Gomez-Munoz, A., O'Brien, L., Hundal, R. & Steinbrecher, U.P. Lysophosphatidylcholine stimulates phospholipase D activity in mouse peritoneal macrophages. J. Lipid Res. 40, 988–993 (1999).
De Vries, H.E. et al. Acute effects of oxidized low density lipoprotein on metabolic responses in macrophages. FASEB J. 12, 111–118 (1998).
Ngwenya, B.Z. & Yamamoto, N. Effects of inflammation products on immune systems. Lysophosphatidylcholine stimulates macrophages. Cancer Immunol. Immunother. 21, 174–182 (1986).
Ramos, M.A. et al. Induction of macrophage VEGF in response to oxidized LDL and VEGF accumulation in human atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 18, 1188–1196 (1998).
McMurray, H.F., Parthasarathy, S. & Steinberg, D. Oxidatively modified low density lipoprotein is a chemoattractant for human T lymphocytes. J. Clin. Invest. 92, 1004–1008 (1993).
Asaoka, Y., Oka, M., Yoshida, K. & Nishizuka, Y. Lysophosphatidylcholine as a possible second messenger synergistic to diacylglycerol and calcium ion for T-lymphocyte activation. Biochem. Biophys. Res. Commun. 178, 1378–1385 (1991).
Nishi, E. et al. Lysophosphatidylcholine increases expression of heparin-binding epidermal growth factor-like growth factor in human T lymphocytes. Circ. Res. 80, 638–644 (1997).
Nishi, E. et al. Lysophosphatidylcholine enhances cytokine-induced interferon gamma expression in human T lymphocytes. Circ. Res. 83, 508–515 (1998).
Asaoka, Y., Oka, M., Yoshida, K., Sasaki, Y. & Nishizuka, Y. Role of lysophosphatidylcholine in T-lymphocyte activation: involvement of phospholipase A2 in signal transduction through protein kinase C. Proc. Natl. Acad. Sci. USA 89, 6447–6451 (1992).
Savage, J.E., Theron, A.J. & Anderson, R. Activation of neutrophil membrane-associated oxidative metabolism by ultraviolet radiation. J. Invest. Dermatol. 101, 532–536 (1993).
Nishioka, H., Horiuchi, H., Arai, H. & Kita, T. Lysophosphatidylcholine generates superoxide anions through activation of phosphatidylinositol 3-kinase in human neutrophils. FEBS Lett. 441, 63–66 (1998).
Silliman, C.C. et al. Lysophosphatidylcholines prime the NADPH oxidase and stimulate multiple neutrophil functions through changes in cytosolic calcium. J. Leukoc. Biol. 73, 511–524 (2003).
Kabarowski, J.H., Zhu, K., Le, L.Q., Witte, O.N. & Xu, Y. Lysophosphatidylcholine as a ligand for the immunoregulatory receptor G2A. Science 293, 702–705 (2001).
Muller Kobold, A.C. et al. Leukocyte activation in sepsis; correlations with disease state and mortality. Intensive Care Med. 26, 883–892 (2000).
Solomkin, J.S., Jenkins, M.K., Nelson, R.D., Chenoweth, D. & Simmons, R.L. Neutrophil dysfunction in sepsis. II. Evidence for the role of complement activation products in cellular deactivation. Surgery 90, 319–327 (1981).
Zhu, K. et al. Sphingosylphosphorylcholine and lysophosphatidylcholine are ligands for the G protein-coupled receptor GPR4. J. Biol. Chem. 276, 41325–41335 (2001).
Okajima, F. et al. Stimulatory and inhibitory actions of lysophosphatidylcholine, depending on its fatty acid residue, on the phospholipase C/Ca2+ system in HL-60 leukaemia cells. Biochem. J. 336, 491–500 (1998).
Zantl, N. et al. Essential role of gamma interferon in survival of colon ascendens stent peritonitis, a novel murine model of abdominal sepsis. Infect. Immun. 66, 2300–2309 (1998).
Tzianabos, A.O. et al. IL-2 mediates protection against abscess formation in an experimental model of sepsis. J. Immunol. 163, 893–897 (1999).
Weighardt, H. et al. Impaired monocyte IL-12 production before surgery as a predictive factor for the lethal outcome of postoperative sepsis. Ann. Surg. 235, 560–567 (2002).
Nakahata, E., Shindoh, Y., Takayama, T. & Shindoh, C. Interleukin-12 prevents diaphragm muscle deterioration in a septic animal model. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 130, 653–663 (2001).
Remick, D.G. et al. Combination immunotherapy with soluble tumor necrosis factor receptors plus interleukin 1 receptor antagonist decreases sepsis mortality. Crit. Care Med. 29, 473–481 (2001).
Matsumoto, T. et al. Contribution of neutrophils to lipopolysaccharide-induced tumor necrosis factor production and mortality in a carrageenan-pretreated mouse model. FEMS Immunol. Med. Microbiol. 17, 171–178 (1997).
Drobnik, W. et al. Plasma ceramide and lysophosphatidylcholine inversely correlate with mortality in sepsis patients. J. Lipid Res. 44, 754–761 (2003).
Macphee, C.H. Lipoprotein-associated phospholipase A2: a potential new risk factor for coronary artery disease and a therapeutic target. Curr. Opin. Pharmacol. 1, 121–125 (2001).
Corr, P.B. et al. Pathophysiological concentrations of lysophosphatides and the slow response. Am. J. Physiol. 243, H187–H195 (1982).
Kishimoto, T., Soda, Y., Matsuyama, Y. & Mizuno, K. An enzymatic assay for lysophosphatidylcholine concentration in human serum and plasma. Clin. Biochem. 35, 411–416 (2002).
Szucs, S., Varga, C., Ember, I. & Kertai, P. The separation of the granulocytes from different rat strains. A comparative study. J. Immunol. Methods 167, 245–251 (1994)
Qureshi, M.A. & Dietert, R.R. Bacterial uptake and killing by macrophages. in Methods in Immunotoxicology Vol. 2 (eds. Burleson, G.R., Dean, J.H. & Munson, A.E.) 119–131 (Wiley-Liss, New York, 1995).
Weng, Z. et al. A DNA damage and stress inducible G protein-coupled receptor blocks cells in G2/M. Proc. Natl. Acad. Sci. USA 95, 12334–12339 (1998).
Acknowledgements
We thank J. Chun for advice and critical reading of the manuscript; J.-B. Kim and J.-I. Kim for comments; and J.-N. Suh, J.-G. Ahn and Y.-M. Kim for technical assistance. This work was supported by Hallym University and the 21st Century Frontier Research Program (M103KV010013 03K2201 01330 to S.-O.H.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
D.-K.S., S.-O.H. and H.-W.S. have over 5% equity in Biosynergen Inc., which has filed a license on the use of lysophosphatidylcholine in sepsis.
Supplementary information
Rights and permissions
About this article
Cite this article
Yan, JJ., Jung, JS., Lee, JE. et al. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat Med 10, 161–167 (2004). https://doi.org/10.1038/nm989
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm989
This article is cited by
-
Distinctly altered lipid components in hepatocellular carcinoma relate to impaired T cell-dependent antitumor immunity
Hepatology International (2023)
-
RETRACTED ARTICLE: Lysophosphatidylcholine induces adenosine release from macrophages via TRPM7-mediated mitochondrial activation
Purinergic Signalling (2022)
-
A metabolomic endotype of bioenergetic dysfunction predicts mortality in critically ill patients with acute respiratory failure
Scientific Reports (2021)
-
The trans-omics landscape of COVID-19
Nature Communications (2021)
-
Lysophosphatidylcholine acyltransferase 2 (LPCAT2) co-localises with TLR4 and regulates macrophage inflammatory gene expression in response to LPS
Scientific Reports (2020)