Abstract
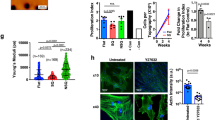
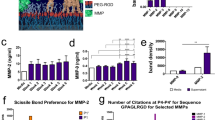
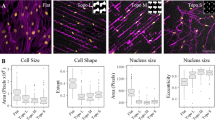
There is currently an unmet need for the supply of autologous, patient-specific stem cells for regenerative therapies in the clinic. Mesenchymal stem cell differentiation can be driven by the material/cell interface suggesting a unique strategy to manipulate stem cells in the absence of complex soluble chemistries or cellular reprogramming. However, so far the derivation and identification of surfaces that allow retention of multipotency of this key regenerative cell type have remained elusive. Adult stem cells spontaneously differentiate in culture, resulting in a rapid diminution of the multipotent cell population and their regenerative capacity. Here we identify a nanostructured surface that retains stem-cell phenotype and maintains stem-cell growth over eight weeks. Furthermore, the study implicates a role for small RNAs in repressing key cell signalling and metabolomic pathways, demonstrating the potential of surfaces as non-invasive tools with which to address the stem cell niche.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Mirmalek-Sani, S. H. et al. Characterization and multipotentiality of human fetal femur-derived cells: Implications for skeletal tissue regeneration. Stem Cells 24, 1042–1053 (2006).
McBeath, R., Pirone, D. M., Nelson, C. M., Bhadriraju, K. & Chen, C. S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 6, 483–495 (2004).
Kilian, K. A., Bugarija, B., Lahn, B. T. & Mrksich, M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl Acad. Sci. USA 107, 4872–4877 (2010).
Curran, J. M., Chen, R. & Hunt, J. A. The guidance of human mesenchymal stem cell differentiation in vitro by controlled modifications to the cell substrate. Biomaterials 27, 4783–4793 (2006).
Benoit, D. S. W., Schwartz, M. P., Durney, A. R. & Anseth, K. S. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nature Mater. 7, 816–823 (2008).
Engler, A. J., Sen, S., Sweeney, H. L. & Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006).
Dalby, M. J. et al. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nature Mater. 6, 997–1003 (2007).
Oh, S. et al. Stem cell fate dictated solely by altered nanotube dimension. Proc. Natl Acad. Sci. USA 106, 2130–2135 (2009).
Yanes, O. et al. Metabolic oxidation regulates embryonic stem cell differentiation. Nature Chem. Biol. 6, 411–417 (2010).
Reyes, J. M. et al. Metabolic changes in mesenchymal stem cells in osteogenic medium measured by autofluorescence spectroscopy. Stem Cells 24, 1213–1217 (2006).
Kuroda, Y. et al. Unique multipotent cells in adult human mesenchymal cell populations. Proc. Natl Acad. Sci. USA 107, 8639–8643 (2010).
Pacini, S. et al. Constitutive expression of pluripotency-associated genes in mesodermal progenitor cells (MPCs). PLoS ONE 5, e9861 (2010).
Kiss-Laszlo, Z., Henry, Y. & Kiss, T. Sequence and structural elements of methylation guide snoRNAs essential for site-specific ribose methylation of pre-rRNA. EMBO J. 17, 797–807 (1998).
Kishore, S. & Stamm, S. Regulation of alternative splicing by snoRNAs. Cold Spring Harb. Symp. Quant. Biol. 71, 329–334 (2006).
Ender, C. et al. A human snoRNA with microRNA-like functions. Mol. Cell 32, 519–528 (2008).
Mattick, J. S. & Makunin, I. V. Small regulatory RNAs in mammals. Human Mol. Genet. 14, R121–R132 (2005).
Gangaraju, V. K. & Lin, H. MicroRNAs: key regulators of stem cells. Nature Rev. Mol. Cell Biol. 10, 116–125 (2009).
Stadler, B. M. & Ruohola-Baker, H. Small RNAs: Keeping stem cells in line. Cell 132, 563–566 (2008).
Cassano, M. et al. Magic-factor 1, a partial agonist of Met, induces muscle hypertrophy by protecting myogenic progenitors from apoptosis. PLoS ONE 3, e3223 (2008).
Kang, Y. J. et al. Role of c-Jun N-terminal kinase in the PDGF-induced proliferation and migration of human adipose tissue-derived mesenchymal stem cells. J. Cell Biochem. 95, 1135–1145 (2005).
Bocelli-Tyndall, C. et al. Fibroblast growth factor 2 and platelet-derived growth factor, but not platelet lysate, induce proliferation-dependent, functional class II major histocompatibility complex antigen in human mesenchymal stem cells. Arthritis Rheum. 62, 3815–3825 (2010).
Sabri, A., Ziaee, A. A., Ostad, S. N., Alimoghadam, K. & Ghahremani, M. H. Crosstalk of EGF-directed MAPK signalling pathways and its potential role on EGF-induced cell proliferation and COX-2 expression in human mesenchymal stem cells. Cell Biochem. Funct. 29, 64–70 (2011).
Kapinas, K., Kessler, C., Ricks, T., Gronowicz, G. & Delany, A. M. miR-29 modulates Wnt signaling in human osteoblasts through a positive feedback loop. J. Biol. Chem. 285, 25221–25231 (2010).
Yang, Z. et al. MicroRNA hsa-miR-138 inhibits adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells through EID-1. Stem Cells Dev. 259–267 (2010).
Xiao, G., Jiang, D., Gopalakrishnan, R. & Franceschi, R. T. Fibroblast growth factor 2 induction of the osteocalcin gene requires MAPK activity and phosphorylation of the osteoblast transcription factor, Cbfa1/Runx2. J. Biol. Chem. 277, 36181–36187 (2002).
Ge, C., Xiao, G., Jiang, D. & Franceschi, R. T. Critical role of the extracellular signal-regulated kinase-MAPK pathway in osteoblast differentiation and skeletal development. J. Cell Biol. 176, 709–718 (2007).
Liao, Q. C. et al. Inhibition of adipocyte differentiation by phytoestrogen genistein through a potential downregulation of extracellular signal-regulated kinases 1/2 activity. J. Cell Biochem. 104, 1853–1864 (2008).
Nakano, N. et al. Gliclazide inhibits proliferation but stimulates differentiation of white and brown adipocytes. J. Biochem. 142, 639–645 (2007).
Wang, N., Tytell, J. D. & Ingber, D. E. Mechanotransduction at a distance: Mechanically coupling the extracellular matrix with the nucleus. Nature Rev. Mol. Cell Biol. 10, 75–82 (2009).
Guilak, F. et al. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 5, 17–26 (2009).
Discher, D. E., Mooney, D. J. & Zandstra, P. W. Growth factors, matrices, and forces combine and control stem cells. Science 324, 1673–1677 (2009).
Kwan, A. P., Cummings, C. E., Chapman, J. A. & Grant, M. E. Macromolecular organization of chicken type X collagen in vitro. J. Cell Biol. 114, 597–604 (1991).
Stephens, M., Kwan, A. P., Bayliss, M. T. & Archer, C. W. Human articular surface chondrocytes initiate alkaline phosphatase and type X collagen synthesis in suspension culture. J. Cell Sci. 103 (Pt 4), 1111–1116 (1992).
Gilbert, P. M. et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329, 1078–1081 (2010).
Gadegaard, N., Mosler, M. & Larsen, M. B. Biomimetic polymer nanostructures by injection molding. Macromol. Mater. Eng. 288, 76–83 (2003).
Acknowledgements
Funding for this work is gratefully acknowledged from the following: A Lord Kelvin/Adam Smith Scholarship for R.J.M. from the University of Glasgow, BBSRC follow-on grant BB/FOF/210 and BBSRC project grant BBG0088681 (to M.J.D. and N.G.). Work from ROCO laboratory is funded through BBSRC BB/G006970/1 and we acknowledge the help of the Sir Henry Welcome Functional Genomics Facility with microarray and bioinformatics, especially P. Herzyk. Bone samples from the orthopaedic surgeons at Southampton General Hospital are gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
R.J.M. performed most of the practical work and was also involved in experimental design, data analysis and writing of the manuscript. N.G. designed, fabricated and characterized the nanoscale patterns, devised imprinting techniques for the polymers and helped write the manuscript. K.V.B. developed metabolite isolation protocols, ran the mass spectroscopy analysis and helped with bioinformatics. P.M.T. performed experiments on polycarbonate and also performed the integrin and cytoskeletal inhibitor studies. E.K. and R.T. performed most of the qPCR and associated data analysis. L.E.M. analysed the small RNA data and helped write the manuscript. K.M. isolated all the STRO-1 cells, performed passaging experiments and analysed the data. R.O.C.O. lead and designed the Southampton experiments, organized many aspects of the work and helped analyse the data and write the manuscript. M.J.D. designed many of the experiments, analysed the data with R.J.M. and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1968 kb)
Rights and permissions
About this article
Cite this article
McMurray, R., Gadegaard, N., Tsimbouri, P. et al. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nature Mater 10, 637–644 (2011). https://doi.org/10.1038/nmat3058
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3058
This article is cited by
-
Non-viral gene delivery to human mesenchymal stem cells: a practical guide towards cell engineering
Journal of Biological Engineering (2023)
-
In situ construction of flower-like nanostructured calcium silicate bioceramics for enhancing bone regeneration mediated via FAK/p38 signaling pathway
Journal of Nanobiotechnology (2022)
-
Cell-controlled dynamic surfaces for skeletal stem cell growth and differentiation
Scientific Reports (2022)
-
Changes in the Number of Mesenchymal Stem Cells in Bone Marrow at Different Periods after In Vivo Exposure of the Bone Marrow to Local Infrared Laser Radiation
Bulletin of Experimental Biology and Medicine (2022)
-
Extracellular Matrix Remodeling and Development of Cancer
Stem Cell Reviews and Reports (2021)