Abstract
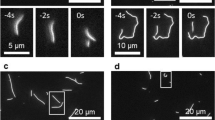
Regulation of microtubule dynamics is essential for many cell biological processes and is likely to be variable between different subcellular regions. We describe a computational approach to analyze microtubule dynamics by detecting growing microtubule plus ends. Our algorithm tracked all EB1-EGFP comets visible in an image time-lapse sequence allowing the detection of spatial patterns of microtubule dynamics. We introduce spatiotemporal clustering of EB1-EGFP growth tracks to infer microtubule behaviors during phases of pause and shortening. We validated the algorithm by comparing the results to data for manually tracked, homogeneously labeled microtubules and by analyzing the effects of well-characterized inhibitors of microtubule polymerization dynamics. We used our method to analyze spatial variations of intracellular microtubule dynamics in migrating epithelial cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Desai, A. & Mitchison, T.J. Microtubule polymerization dynamics. Annu. Rev. Cell Dev. Biol. 13, 83–117 (1997).
Sammak, P.J. & Borisy, G.G. Direct obsrevation of microtubule dynamics in living cells. Nature 332, 724–726 (1988).
Rusan, N.M., Fagerstrom, C.J., Yvon, A.M.C. & Wadsworth, P. Cell cycle-dependent changes in microtubule dynamics in living cells expressing green fluorescent protein-alpha tubulin. Mol. Biol. Cell 12, 971–980 (2001).
Wittmann, T., Bokoch, G.M. & Waterman-Storer, C.M. Regulation of leading edge microtubule and actin dynamics downstream of Rac1. J. Cell Biol. 161, 845–851 (2003).
Altinok, A. et al. Model based dynamics analysis in live cell microtubule images. BMC Cell Biol. 8 (Suppl. 1), S4 (2007).
Waterman-Storer, C.M. & Salmon, E.D. How microtubules get fluorescent speckles. Biophys. J. 75, 2059–2069 (1998).
Salmon, W.C., Adams, M.C. & Waterman-Storer, C.M. Dual-wavelength fluorescent speckle microscopy reveals coupling of microtubule and actin movements in migrating cells. J. Cell Biol. 158, 31–37 (2002).
Akhmanova, A. & Steinmetz, M.O. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat. Rev. Mol. Cell Biol. 9, 309–322 (2008).
Salaycik, K.J., Fagerstrom, C.J., Murthy, K., Tulu, U.S. & Wadsworth, P. Quantification of microtubule nucleation, growth and dynamics in wound-edge cells. J. Cell Sci. 118, 4113–4122 (2005).
Bieling, P. et al. CLIP-170 tracks growing microtubule ends by dynamically recognizing composite EB1/tubulin-binding sites. J. Cell Biol. 183, 1223–1233 (2008).
Dragestein, K.A. et al. Dynamic behavior of GFP-CLIP-170 reveals fast protein turnover on microtubule plus ends. J. Cell Biol. 180, 729–737 (2008).
Rosin, P.L. Unimodal thresholding. Pattern Recognit. 34, 2083–2096 (2001).
Yang, G., Matov, A. & Danuser, G. Reliable tracking of large-scale dense particle motion for fluorescent live cell imaging. IEEE Int. Conf. Comp. Vis. Patt. Rec. 3, 138 (2005).
Verde, F., Dogterom, M., Stelzer, E., Karsenti, E. & Leibler, S. Control of microtunbule dynamics and length by cyclin A-dependent and cyclin B-dependent kinases in Xenopus egg extracts. J. Cell Biol. 118, 1097–1108 (1992).
Hawkins, T., Mirigian, M., Yasar, M.S. & Ross, J.L. Mechanics of microtubules. J. Biomech. 43, 23–30 (2010).
Brangwynne, C.P., MacKintosh, F.C. & Weitz, D.A. Force fluctuations and polymerization dynamics of intracellular microtubules. Proc. Natl. Acad. Sci. USA 104, 16128–16133 (2007).
Schrijver, A. Combinatorial Optimization. (Springer, Heidelberg, 2003).
Jaqaman, K. et al. Robust single-particle tracking in live-cell time-lapse sequences. Nat. Methods 5, 695–702 (2008).
Vasquez, R.J., Howell, B., Yvon, A.M.C., Wadsworth, P. & Cassimeris, L. Nanomolar concentrations of nocodazole alter microtubule dynamic instability in vivo and in vitro. Mol. Biol. Cell 8, 973–985 (1997).
Yvon, A.M.C., Wadsworth, P. & Jordan, M.A. Taxol suppresses dynamics of individual microtubules in living human tumor cells. Mol. Biol. Cell 10, 947–959 (1999).
Hammond, J.W., Cai, D.W. & Verhey, K.J. Tubulin modifications and their cellular functions. Curr. Opin. Cell Biol. 20, 71–76 (2008).
Hubbert, C. et al. HDAC6 is a microtubule-associated deacetylase. Nature 417, 455–458 (2002).
Komarova, Y.A., Vorobjev, I.A. & Borisy, G.G. Life cycle of MTs: persistent growth in the cell interior, asymmetric transition frequencies and effects of the cell boundary. J. Cell Sci. 115, 3527–3539 (2002).
Kumar, P. et al. GSK3 beta phosphorylation modulates CLASP-microtubule association and lamella microtubule attachment. J. Cell Biol. 184, 895–908 (2009).
Mimori-Kiyosue, Y. et al. CLASP1 and CLASP2 bind to EB1 and regulate microtubule plus-end dynamics at the cell cortex. J. Cell Biol. 168, 141–153 (2005).
Vitre, B. et al. EB1 regulates microtubule dynamics and tubulin sheet closure in vitro. Nat. Cell Biol. 10, 415–421 (2008).
Komarova, Y. et al. Mammalian end binding proteins control persistent microtubule growth. J. Cell Biol. 184, 691–706 (2009).
Skube, S.B., Chaverri, J.M., & Goodson, H.V. Effect of GFP tags on the localization of EB1 and EB1 fragments in vivo. Cytoskeleton 67, 1–12 (2010).
Honnappa, S. et al. An EB1-binding motif acts as a microtubule tip localization signal. Cell 138, 366–376 (2009).
Jaqaman, K. et al. Kinetochore alignment within the metaphase plate is regulated by centromere stiffness and microtubule depolymerases. J. Cell Biol. 188, 665–679 (2010).
Acknowledgements
This work was supported by US National Institutes of Health grants U01 GM067230 to G.D. and R01 GM079139 to T.W. This research was in part conducted in a facility constructed with support from the Research Facilities Improvement Program grant C06 RR16490 from the National Center for Research Resources of the National Institutes of Health. We thank A. Wheeler (Imperial College London) for the EB1-EGFP–expressing HaCaT cell line.
Author information
Authors and Affiliations
Contributions
A.M. designed and implemented algorithms for comet detection and track clustering, and performed computer vision and statistical analysis. P.K. and T.W. acquired EB1-EGFP and mCherry-tubulin datasets. C.T. and W.K. generated data for the comparison of EB3-EGFP and CLIP170-EGFP comet dynamics in pVHL knockdown cells. G.D. conceived the algorithm for growth-track clustering and designed validation experiments. T.W. directed image acquisition and contributed to data analysis. All authors contributed to the interpretation of the results and to the discussion of improvements to the software. G.D., T.W. and A.M. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6, Supplementary Tables 1–2 and Supplementary Notes 1–9 (PDF 1679 kb)
Supplementary Software 1
ClusterTrack software for detection, tracking and growth track clustering of plus end-labeled microtubules in the analysis of microtubule dynamics. (ZIP 143932 kb)
Supplementary Video 1
Overlay of computer-generated growth tracks (yellow) onto a EB1-EGFP time-lapse sequence. Total length is 30 s (75 frames). Images were acquired every 0.4 s. Video plays at 15 frames s−1, and is thus accelerated 6×. (MOV 5518 kb)
Supplementary Video 2
Dual-wavelength time-lapse sequence of mCherry-tubulin and EB1-EGFP used for validation of the clustering algorithm. Hand-tracked microtubule ends are indicated by blue crosses. Total length is 60 s (97 frames). Images were acquired every 0.62 s. Video plays at 15 frames s−1, and is thus accelerated 9.3×. (MOV 7161 kb)
Supplementary Video 3
Same sequence as Supplementary Video 2 with automatically detected EB1-EGFP comets highlighted by bright green dots. (MOV 6352 kb)
Supplementary Video 4
Region of the dual-wavelength time-lapse sequence in Supplementary Video 2 showing examples of microtubule trajectories inferred by the clustering algorithm. Shown are the microtubule channel (top left), EB1 channel (bottom left), microtubule and EB1 color overlay (top right), and microtubule trajectory overlaid on the EB1 channel (bottom right). Green indicates growth tracks, and red are backward and blue are forward gaps. Such videos were used for the visual validation documented in Supplementary Table 2. This video displays a trajectory deemed as correct. (MOV 4968 kb)
Supplementary Video 5
Second example of a probably correct computer-inferred microtubule trajectory. Different channels and color-coded lines are as indicated for Supplementary Video 4. Note that the growth phase before the last backward gap was tracked as too short because of the low signal-to-noise ratio of the EB1 comet and its partial overlap with another comet. This inaccuracy will lead to an underestimation of the inferred shortening speed and overestimation of the inferred catastrophe rate for this specific trajectory. However, because all phases are assigned properly, this trajectory does not produce a false positive or a false negative. (MOV 6882 kb)
Supplementary Video 6
Example of a computer-inferred microtubule trajectory probably containing a false positive growth event at the end of the trajectory. The trajectory appears to switch to a different microtubule during the last forward gap. Different channels and color-coded lines are as indicated for Supplementary Video 4. (MOV 4636 kb)
Rights and permissions
About this article
Cite this article
Matov, A., Applegate, K., Kumar, P. et al. Analysis of microtubule dynamic instability using a plus-end growth marker. Nat Methods 7, 761–768 (2010). https://doi.org/10.1038/nmeth.1493
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.1493
This article is cited by
-
Microtubules as a potential platform for energy transfer in biological systems: a target for implementing individualized, dynamic variability patterns to improve organ function
Molecular and Cellular Biochemistry (2023)
-
2D-GolgiTrack—a semi-automated tracking system to quantify morphological changes and dynamics of the Golgi apparatus and Golgi-derived membrane tubules
Medical & Biological Engineering & Computing (2022)
-
Imaging mitotic processes in three dimensions with lattice light-sheet microscopy
Chromosome Research (2021)
-
In vivo microscopy reveals macrophage polarization locally promotes coherent microtubule dynamics in migrating cancer cells
Nature Communications (2020)
-
SUGT1 controls susceptibility to HIV-1 infection by stabilizing microtubule plus-ends
Cell Death & Differentiation (2020)