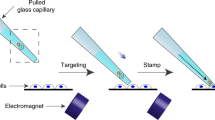
Abstract
Introduction of exogenous DNA into mammalian cells represents a powerful approach for manipulating signal transduction. The available techniques, however, are limited by low transduction efficiency and low cell viability after transduction. Here we report a highly efficient molecular delivery technique, named nanotube spearing, based on the penetration of nickel-embedded nanotubes into cell membranes by magnetic field driving. DNA plasmids containing the enhanced green fluorescent protein (EGFP) sequence were immobilized onto the nanotubes, and subsequently speared into targeted cells. We have achieved an unprecedented high transduction efficiency in Bal17 B-lymphoma, ex vivo B cells and primary neurons with high viability after transduction. This technique may provide a powerful tool for highly efficient gene transfer into a variety of cells, especially the hard-to-transfect cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ren, Z.F. et al. Synthesis of large arrays of well-aligned carbon nanotubes on glass. Science 282, 1105–1107 (1998).
Wen, J.G. et al. Growth and characterization of aligned carbon nanotubes from patterned nickel nanodots and uniform thin films. J. Mater. Res. 16, 3246–3253 (2001).
Pantarotto, D., Briand, J.P., Prato, M. & Bianco, A. Translocation of bioactive peptides across cell membranes by carbon nanotubes. Chem. Comm. 1, 16–17 (2004).
Shi Kam, N.W., Jessop, T.C., Wender, P.A. & Dai, H. Nanotube molecular transporters: internalization of carbon nanotube-protein conjugates into mammalian cells. J. Am. Chem. Soc. 126, 6850–6851 (2004).
Hu, H., Ni, Y., Montana, V., Haddon, R.C. & Parpura, V. Chemically functionalized carbon nanotubes as substrates for neuronal growth. Nano Lett. 4, 507–511 (2004).
McKnight, T.E. et al. Intracellular integration of synthetic nanostructures with viable cells for controlled biochemical manipulation. Nanotechnology 14, 551–556 (2003).
Klein, T.M., Arentzen, R., Lewis, P.A. & Fitzpatrick-McElligott, S. Transformation of microbes, plants and animals by particle bombardment. Biotechnology 10, 286–291 (1992).
Shen, H., Tan, J. & Saltzman, W.M. Surface-mediated gene transfer from nanocomposites of controlled texture. Nat. Mater. 3, 569–574 (2004).
Salem, A.K., Searson, P.C. & Leong, K.W. Multifunctional nanorods for gene delivery. Nat. Mater. 2, 668–671 (2003).
Pampinella, F. et al. Analysis of differential lipofection efficiency in primary and established myoblasts. Mol. Ther. 5, 161–169 (2002).
Cheng, R.Y. et al. Gene expression dose-response changes in microarrays after exposure of human peripheral lung epithelial cells to nickel(II). Toxic Appl. Pharmacol. 191, 22–39 (2003).
Williams, K.A., Veenhuizen, P.T.M., de la Torre, B.G., Eritjia, R. & Dekker, C. Nanotechnology: carbon nanotubes with DNA recognition. Nature 420, 761 (2002).
Hazani, M., Naaman, R., Hennrich, F. & Kappes, M.M. Confocal fluorescence imaging of DNA-functionalized carbon nanotubes. Nano Lett. 3, 153–155 (2003).
Tumang, J.R., Hastings, W.D., Bai, C. & Rothstein, T.L. Peritoneal and splenic B-1 cells are separable by phenotypic, functional, and transcriptomic characteristics. Eur. J. Immunol. 34, 2158–2167 (2004).
Amato, S.F., Nakajima, K., Hirano, T. & Chiles, T.C. Transcriptional regulation of the junB promoter in mature B lymphocytes. Activation through a cyclic adenosine 3′,5′-monophosphate- like binding site. J. Immunol. 157, 146–155 (1996).
Hamm, A., Krott, N., Breibach, I., Blindt, R. & Bosserhoff, A.K. Efficient transfection method for primary cells. Tissue Eng. 8, 235–245 (2002).
Bigey, P., Bureau, M.F. & Scherman, D. In vivo plasmid DNA electrotransfer. Curr. Opin. Biotechnol. 13, 443–447 (2002).
McMahon, J.M. & Wells, D.J. Electroportaion for gene transfer to skeletal muscles: current status. BioDrugs 18, 155–165 (2004).
McMahon, J.M. et al. Optimisation of electrotransfer of plasmid into skeletal muscle by pre-treatment with hyaluronidase-increased expression with reduced muscle damage. Gene Ther. 8, 1264–1270 (2001).
Durieux, A.C., Bonnefoy, R., Busso, T. & Freyssenet, D. In vivo gene electrotransfer into skeletal muscle: effects of plasmid DNA on the occurrence and extent of muscle damage. J. Gene Med. 6, 809–816 (2003).
Wells, D.J. Gene therapy progress and prospects: electroporation and other physical methods. Gene Ther. 11, 1363–1369 (2004).
Scherer, F. et al. Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 9, 102–109 (2002).
Obataya, I., Nakamura, C., Han, S., Nakamura, N. & Miyake, J. Nanoscale operation of a living cell using an atomic force microscope with a nanoneedle. Nano Lett. 5, 27–30 (2005).
Huang, Z.P. et al. Effect of nickel, iron, and cobalt on growth of aligned carbon nanotubes. Appl. Phys. A74, 387–391 (2002).
Rizk, N.N. & El-Rakhawy, M.T. Tissue culture and scanning electron microscopy of breast carcinoma 'cell line MCF-7' from pleural effusion. Acta Anat. 109, 70–74 (1981).
Acknowledgements
The work performed at Boston College is partly supported by the US Department of Energy (DE-FG02-00ER45805), National Science Foundation (NIRT 0304506), and National Institutes of Health (Public Health Service Grant AI-34586). We thank D. Burgess (Boston College, Department of Biology) for the EGFP expressing plasmid. We also thank members of the Burgess' laboratory and Department of Biology for allowing us to use the Confocal Microscopy Facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Nanolab, Inc. is planning to commercialize the technique described in this article.
Supplementary information
Supplementary Fig. 1
Carbon nanotube and Ni particle morphology. (PDF 210 kb)
Supplementary Fig. 2
Cell mortality after nanotube spearing. (PDF 60 kb)
Supplementary Fig. 3
The layers of graphene sheets and/or amorphous carbon that enclose the Ni particle in the carbon nanotubes. (PDF 82 kb)
Supplementary Fig. 4
The Ni particle deprived nanotube. (PDF 93 kb)
Supplementary Fig. 5
Nuclei staining with DAPI in cortical neurons 72 hours after spearing. (PDF 177 kb)
Supplementary Fig. 6
EGFP transfection with Lipofectamine 2000 in Bal17 cells. (PDF 279 kb)
Supplementary Fig. 7
EGFP transfection with Lipofectamine 2000 in primary B cells. (PDF 324 kb)
Rights and permissions
About this article
Cite this article
Cai, D., Mataraza, J., Qin, ZH. et al. Highly efficient molecular delivery into mammalian cells using carbon nanotube spearing. Nat Methods 2, 449–454 (2005). https://doi.org/10.1038/nmeth761
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth761
This article is cited by
-
Wettability and confinement size effects on stability of water conveying nanotubes
Scientific Reports (2020)
-
Gold-decorated sulfur-doped carbon nanotubes as electrocatalyst in hydrogen evolution reaction
Gold Bulletin (2020)
-
RETRACTED ARTICLE: The structure and biological properties of clustered anatase/rutile nanowire array–modified titanium surface
Journal of Nanoparticle Research (2020)