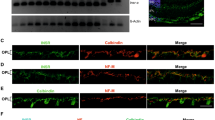
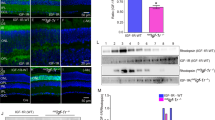
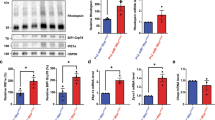
Abstract
Retinitis pigmentosa is an incurable retinal disease that leads to blindness. One puzzling aspect concerns the progression of the disease. Although most mutations that cause retinitis pigmentosa are in rod photoreceptor–specific genes, cone photoreceptors also die as a result of such mutations. To understand the mechanism of non-autonomous cone death, we analyzed four mouse models harboring mutations in rod-specific genes. We found changes in the insulin/mammalian target of rapamycin pathway that coincided with the activation of autophagy during the period of cone death. We increased or decreased the insulin level and measured the survival of cones in one of the models. Mice that were treated systemically with insulin had prolonged cone survival, whereas depletion of endogenous insulin had the opposite effect. These data suggest that the non-autonomous cone death in retinitis pigmentosa could, at least in part, be a result of the starvation of cones.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Steinberg, R.H. Survival factors in retinal degenerations. Curr. Opin. Neurobiol. 4, 515–524 (1994).
Mohand-Said, S. et al. Normal retina releases a diffusible factor stimulating cone survival in the retinal degeneration mouse. Proc. Natl. Acad. Sci. USA 95, 8357–8362 (1998).
Streichert, L.C., Birnbach, C.D. & Reh, T.A. A diffusible factor from normal retinal cells promotes rod photoreceptor survival in an in vitro model of retinitis pigmentosa. J. Neurobiol. 39, 475–490 (1999).
Mohand-Said, S. et al. Photoreceptor transplants increase host cone survival in the retinal degeneration (rd) mouse. Ophthalmic Res. 29, 290–297 (1997).
Mohand-Said, S., Hicks, D., Dreyfus, H. & Sahel, J.A. Selective transplantation of rods delays cone loss in a retinitis pigmentosa model. Arch. Ophthalmol. 118, 807–811 (2000).
Leveillard, T. et al. Identification and characterization of rod-derived cone viability factor. Nat. Genet. 36, 755–759 (2004).
Gupta, N., Brown, K.E. & Milam, A.H. Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration and age-related macular degeneration. Exp. Eye Res. 76, 463–471 (2003).
Komeima, K., Rogers, B.S., Lu, L. & Campochiaro, P.A. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 103, 11300–11305 (2006).
Komeima, K., Rogers, B.S. & Campochiaro, P.A. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J. Cell Physiol. 213, 809–815 (2007).
Yu, D.Y. & Cringle, S.J. Retinal degeneration and local oxygen metabolism. Exp. Eye Res. 80, 745–751 (2005).
Bowes, C. et al. Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP-phosphodiesterase. Nature 347, 677–680 (1990).
Tsang, S.H. et al. Retinal degeneration in mice lacking the gamma subunit of the rod cGMP phosphodiesterase. Science 272, 1026–1029 (1996).
Lem, J. et al. Morphological, physiological, and biochemical changes in rhodopsin knockout mice. Proc. Natl. Acad. Sci. USA 96, 736–741 (1999).
Naash, M.I., Hollyfield, J.G., al-Ubaidi, M.R. & Baehr, W. Simulation of human autosomal dominant retinitis pigmentosa in transgenic mice expressing a mutated murine opsin gene. Proc. Natl. Acad. Sci. USA 90, 5499–5503 (1993).
Applebury, M.L. et al. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron 27, 513–523 (2000).
John, S.K., Smith, J.E., Aguirre, G.D. & Milam, A.H. Loss of cone molecular markers in rhodopsin-mutant human retinas with retinitis pigmentosa. Mol. Vis. 6, 204–215 (2000).
Reiling, J.H. & Sabatini, D.M. Stress and mTORture signaling. Oncogene 25, 6373–6383 (2006).
Dekanty, A., Lavista-Llanos, S., Irisarri, M., Oldham, S. & Wappner, P. The insulin-PI3K/TOR pathway induces a HIF-dependent transcriptional response in Drosophila by promoting nuclear localization of HIF-alpha/Sima. J. Cell Sci. 118, 5431–5441 (2005).
Hudson, C.C. et al. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol. Cell. Biol. 22, 7004–7014 (2002).
Treins, C., Giorgetti-Peraldi, S., Murdaca, J., Semenza, G.L. & Van Obberghen, E. Insulin stimulates hypoxia-inducible factor 1 through a phosphatidylinositol 3-kinase/target of rapamycin–dependent signaling pathway. J. Biol. Chem. 277, 27975–27981 (2002).
Zhong, H. et al. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 60, 1541–1545 (2000).
Thomas, G.V. et al. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat. Med. 12, 122–127 (2006).
Wang, G.L., Jiang, B.H., Rue, E.A. & Semenza, G.L. Hypoxia-inducible factor 1 is a basic helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 92, 5510–5514 (1995).
Ebert, B.L., Firth, J.D. & Ratcliffe, P.J. Hypoxia and mitochondrial inhibitors regulate expression of glucose transporter 1 via distinct Cis-acting sequences. J. Biol. Chem. 270, 29083–29089 (1995).
Massey, A., Kiffin, R. & Cuervo, A.M. Pathophysiology of chaperone-mediated autophagy. Int. J. Biochem. Cell Biol. 36, 2420–2434 (2004).
Finn, P.F. & Dice, J.F. Proteolytic and lipolytic responses to starvation. Nutrition 22, 830–844 (2006).
Codogno, P. & Meijer, A.J. Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ. 12 Suppl 2: 1509–1518 (2005).
Dice, J.F. Chaperone-mediated autophagy. Autophagy 3, 295–299 (2007).
Kunchithapautham, K. & Rohrer, B. Apoptosis and autophagy in photoreceptors exposed to oxidative stress. Autophagy 3, 433–441 (2007).
Kabeya, Y. et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19, 5720–5728 (2000).
Mizushima, N., Yamamoto, A., Matsui, M., Yoshimori, T. & Ohsumi, Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol. Biol. Cell 15, 1101–1111 (2004).
Punzo, C. & Cepko, C.L. Ultrasound-guided in utero injections allow studies of the development and function of the eye. Dev. Dyn. 237, 1034–1042 (2008).
Cuervo, A.M. & Dice, J.F. Regulation of lamp2a levels in the lysosomal membrane. Traffic 1, 570–583 (2000).
Kiffin, R., Christian, C., Knecht, E. & Cuervo, A.M. Activation of chaperone-mediated autophagy during oxidative stress. Mol. Biol. Cell 15, 4829–4840 (2004).
Cuervo, A.M. & Dice, J.F. Unique properties of lamp2a compared to other lamp2 isoforms. J. Cell Sci. 113, 4441–4450 (2000).
Corrochano, S. et al. Attenuation of vision loss and delay in apoptosis of photoreceptors induced by proinsulin in a mouse model of retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 49, 4188–4194 (2008).
Poitry-Yamate, C.L., Poitry, S. & Tsacopoulos, M. Lactate released by Muller glial cells is metabolized by photoreceptors from mammalian retina. J. Neurosci. 15, 5179–5191 (1995).
Tsacopoulos, M., Poitry-Yamate, C.L., MacLeish, P.R. & Poitry, S. Trafficking of molecules and metabolic signals in the retina. Prog. Retin. Eye Res. 17, 429–442 (1998).
Snodderly, D.M., Sandstrom, M.M., Leung, I.Y., Zucker, C.L. & Neuringer, M. Retinal pigment epithelial cell distribution in central retina of rhesus monkeys. Invest. Ophthalmol. Vis. Sci. 43, 2815–2818 (2002).
Young, R.W. The renewal of rod and cone outer segments in the rhesus monkey. J. Cell Biol. 49, 303–318 (1971).
Biel, M. et al. Selective loss of cone function in mice lacking the cyclic nucleotide–gated channel CNG3. Proc. Natl. Acad. Sci. USA 96, 7553–7557 (1999).
Yang, R.B. et al. Disruption of a retinal guanylyl cyclase gene leads to cone-specific dystrophy and paradoxical rod behavior. J. Neurosci. 19, 5889–5897 (1999).
Stearns, G., Evangelista, M., Fadool, J.M. & Brockerhoff, S.E. A mutation in the cone-specific pde6 gene causes rapid cone photoreceptor degeneration in zebrafish. J. Neurosci. 27, 13866–13874 (2007).
Gouras, P., Kjeldbye, H. & Zack, D.J. Reporter gene expression in cones in transgenic mice carrying bovine rhodopsin promoter/LacZ transgenes. Vis. Neurosci. 11, 1227–1231 (1994).
Woodford, B.J., Chen, J. & Simon, M.I. Expression of rhodopsin promoter transgene product in both rods and cones. Exp. Eye Res. 58, 631–635 (1994).
al-Ubaidi, M.R. et al. Mouse opsin. Gene structure and molecular basis of multiple transcripts. J. Biol. Chem. 265, 20563–20569 (1990).
Wang, Y. et al. A locus control region adjacent to the human red and green visual pigment genes. Neuron 9, 429–440 (1992).
Punzo, C. & Cepko, C. Cellular responses to photoreceptor death in the rd1 mouse model of retinal degeneration. Invest. Ophthalmol. Vis. Sci. 48, 849–857 (2007).
Harding, E.F. An efficient, minimal-storage procedure for calculating the Mann-Whitney U, generalized U and similar distributions. Appl. Stat. 33, 1–6 (1984).
Molday, R.S. & MacKenzie, D. Monoclonal antibodies to rhodopsin: characterization, cross-reactivity and application as structural probes. Biochemistry 22, 653–660 (1983).
Acknowledgements
We thank J. Nathans for the cone-lacZ mouse line and the blue-cone opsin antibody. We are grateful to S. Tsang, M. Naash and J. Lem for the Pde6b−/−, P23H and Rho−/− mice, respectively. The LAMP-2 antibody, developed by B. Granger, was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the US National Institute of Child Health and Human Development and maintained by the University of Iowa. We thank J. Trimarchi, R. Kanadia, N. Perrimon, J. Zirin, M. Feany and C. Tabin for critical reading of the manuscript. This work was supported by the US National Institutes of Health (RO1 EY014466), Macular Vision Research Foundation, Foundation for Retinal Research, Howard Hughes Medical Institute, Merck and by an EMBO fellowship to C.P.
Author information
Authors and Affiliations
Contributions
C.P. conducted the experiments and wrote the manuscript. K.K. performed computational microarray analysis. C.L.C. supervised the project and wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Table 1 (PDF 1906 kb)
Rights and permissions
About this article
Cite this article
Punzo, C., Kornacker, K. & Cepko, C. Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat Neurosci 12, 44–52 (2009). https://doi.org/10.1038/nn.2234
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2234
This article is cited by
-
Soluble CX3CL1-expressing retinal pigment epithelium cells protect rod photoreceptors in a mouse model of retinitis pigmentosa
Stem Cell Research & Therapy (2023)
-
Separate lifetime signatures of macaque S cones, M/L cones, and rods observed with adaptive optics fluorescence lifetime ophthalmoscopy
Scientific Reports (2023)
-
Phagocytosis in the retina promotes local insulin production in the eye
Nature Metabolism (2023)
-
Mathematical model for glutathione dynamics in the retina
Scientific Reports (2023)
-
Lactate-dependent transcriptional regulation controls mammalian eye morphogenesis
Nature Communications (2023)