Abstract
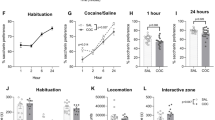
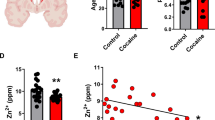
Cocaine addiction is characterized by an impaired ability to develop adaptive behaviors that can compete with cocaine seeking, implying a deficit in the ability to induce plasticity in cortico-accumbens circuitry crucial for regulating motivated behavior. We found that rats withdrawn from cocaine self-administration had a marked in vivo deficit in the ability to develop long-term potentiation (LTP) and long-term depression (LTD) in the nucleus accumbens core subregion after stimulation of the prefrontal cortex. N-acetylcysteine (NAC) treatment prevents relapse in animal models and craving in humans by activating cystine-glutamate exchange and thereby stimulating extrasynaptic metabotropic glutamate receptors (mGluR). NAC treatment of rats restored the ability to induce LTP and LTD by indirectly stimulating mGluR2/3 and mGluR5, respectively. Our findings show that cocaine self-administration induces metaplasticity that inhibits further induction of synaptic plasticity, and this impairment can be reversed by NAC, a drug that also prevents relapse.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Mendelson, J.H. & Mello, N.K. Management of cocaine abuse and dependence. N. Engl. J. Med. 334, 965–972 (1996).
Kalivas, P.W. & O'Brien, C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology 33, 166–180 (2008).
Robinson, T.E. & Kolb, B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology 47 Suppl 1: 33–46 (2004).
Toda, S., Shen, H.W., Peters, J., Cagle, S. & Kalivas, P.W. Cocaine increases actin cycling: effects in the reinstatement model of drug seeking. J. Neurosci. 26, 1579–1587 (2006).
Conrad, K.L. et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 454, 118–121 (2008).
Martin, M., Chen, B.T., Hopf, F.W., Bowers, M.S. & Bonci, A. Cocaine self-administration selectively abolishes LTD in the core of the nucleus accumbens. Nat. Neurosci. 9, 868–869 (2006).
Abraham, W.C. Metaplasticity: tuning synapses and networks for plasticity. Nat. Rev. Neurosci. 9, 387–399 (2008).
Brebner, K. et al. Nucleus accumbens long-term depression and the expression of behavioral sensitization. Science 310, 1340–1343 (2005).
Kauer, J.A. & Malenka, R.C. Synaptic plasticity and addiction. Nat. Rev. Neurosci. 8, 844–858 (2007).
Bamford, N.S. et al. Repeated exposure to methamphetamine causes long-lasting presynaptic corticostriatal depression that is renormalized with drug readministration. Neuron 58, 89–103 (2008).
Kourrich, S., Rothwell, P.E., Klug, J.R. & Thomas, M.J. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J. Neurosci. 27, 7921–7928 (2007).
Madayag, A. et al. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J. Neurosci. 27, 13968–13976 (2007).
Zhou, W. & Kalivas, P.W. N-acetylcysteine reduces extinction responding and induces enduring reductions in cue- and heroin-induced drug-seeking. Biol. Psychiatry 63, 338–340 (2008).
Moran, M.M., McFarland, K., Melendez, R.I., Kalivas, P.W. & Seamans, J.K. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J. Neurosci. 25, 6389–6393 (2005).
Baker, D.A. et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat. Neurosci. 6, 743–749 (2003).
LaRowe, S.D. et al. Is cocaine desire reduced by N-acetylcysteine? Am. J. Psychiatry 164, 1115–1117 (2007).
Goldstein, R.Z. & Volkow, N.D. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychiatry 159, 1642–1652 (2002).
van Dongen, Y.C., Mailly, P., Thierry, A.M., Groenewegen, H.J. & Deniau, J.M. Three-dimensional organization of dendrites and local axon collaterals of shell and core medium-sized spiny projection neurons of the rat nucleus accumbens. Brain Struct. Funct. 213, 129–147 (2008).
Sesack, S.R., Deutch, A.Y., Roth, R.H. & Bunney, B.S. Topographical organization of the efferent projections of the medial prefrontal cortex in rat: An anterograde tract-tracing study with Phaseolus vulgais leucoagglutinin. J. Comp. Neurol. 290, 213–242 (1989).
Nicola, S.M., Kombian, S.B. & Malenka, R.C. Psychostimulants depress excitatory synaptic transmission in the nucleus accumbens via presynaptic D1-like dopamine receptors. J. Neurosci. 16, 1591–1604 (1996).
Pennartz, C.M., Boeijinga, P.H. & Lopes da Silva, F.H. Locally evoked potentials in slices of the rat nucleus accumbens: NMDA and non-NMDA receptor–mediated components and modulation by GABA. Brain Res. 529, 30–41 (1990).
Montaron, M.F., Deniau, J.M., Menetrey, A., Glowinski, J. & Thierry, A.M. Prefrontal cortex inputs of the nucleus accumbens-nigro-thalamic circuit. Neuroscience 71, 371–382 (1996).
Finch, D.M. Neurophysiology of converging synaptic inputs from the rat prefrontal cortex, amygdala, midline thalamus, and hippocampal formation onto single neurons of the caudate/putamen and nucleus accumbens. Hippocampus 6, 495–512 (1996).
Pierce, R.C., Bell, K., Duffy, P. & Kalivas, P.W. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J. Neurosci. 16, 1550–1560 (1996).
Rozas, C. et al. Developmental inhibitory gate controls the relay of activity to the superficial layers of the visual cortex. J. Neurosci. 21, 6791–6801 (2001).
Pennartz, C.M.A., Ameerun, R.F., Groenewegen, H.J. & Lopes da Silva, F.H. Synaptic plasticity in an in vitro slice preparation of the rat nucleus accumbens. Eur. J. Neurosci. 5, 107–117 (1993).
Malenka, R.C. & Bear, M.F. LTP and LTD: an embarrassment of riches. Neuron 44, 5–21 (2004).
Robbe, D., Kopf, M., Remaury, A., Bockaert, J. & Manzoni, O.J. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc. Natl. Acad. Sci. USA 99, 8384–8388 (2002).
Fourgeaud, L. et al. A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. J. Neurosci. 24, 6939–6945 (2004).
Kau, K.S. et al. Blunted cystine-glutamate antiporter function in the nucleus accumbens promotes cocaine-induced drug seeking. Neuroscience 155, 530–537 (2008).
Baptista, M.A., Martin-Fardon, R. & Weiss, F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J. Neurosci. 24, 4723–4727 (2004).
Peters, J. & Kalivas, P.W. The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology (Berl.) 186, 143–149 (2006).
Bossert, J.M., Gray, S.M., Lu, L. & Shaham, Y. Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology 31, 2197–2209 (2006).
McFarland, K., Lapish, C.C. & Kalivas, P.W. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J. Neurosci. 23, 3531–3537 (2003).
Grueter, B.A. & Winder, D.G. Group II and III metabotropic glutamate receptors suppress excitatory synaptic transmission in the dorsolateral bed nucleus of the stria terminalis. Neuropsychopharmacology 30, 1302–1311 (2005).
Losonczy, A., Somogyi, P. & Nusser, Z. Reduction of excitatory postsynaptic responses by persistently active metabotropic glutamate receptors in the hippocampus. J. Neurophysiol. 89, 1910–1919 (2003).
Wu, J., Rowan, M.J. & Anwyl, R. An NMDAR-independent LTP mediated by group II metabotropic glutamate receptors and p42/44 MAP kinase in the dentate gyrus in vitro. Neuropharmacology 46, 311–317 (2004).
Grover, L.M. & Yan, C. Evidence for involvement of group II/III metabotropic glutamate receptors in NMDA receptor–independent long-term potentiation in area CA1 of rat hippocampus. J. Neurophysiol. 82, 2956–2969 (1999).
Backstrom, P. & Hyytia, P. Ionotropic and metabotropic glutamate receptor antagonism attenuates cue-induced cocaine seeking. Neuropsychopharmacology 31, 778–786 (2006).
McGeehan, A.J. & Olive, M.F. The mGluR5 antagonist MPEP reduces the conditioned rewarding effects of cocaine but not other drugs of abuse. Synapse 47, 240–242 (2003).
Lindsley, C.W. et al. Discovery of positive allosteric modulators for the metabotropic glutamate receptor subtype 5 from a series of N-(1,3-diphenyl-1H- pyrazol-5-yl)benzamides that potentiate receptor function in vivo. J. Med. Chem. 47, 5825–5828 (2004).
Yin, H.H., Knowlton, B.J. & Balleine, B.W. Inactivation of dorsolateral striatum enhances sensitivity to changes in the action-outcome contingency in instrumental conditioning. Behav. Brain Res. 166, 189–196 (2006).
Suto, N., Tanabe, L.M., Austin, J.D., Creekmore, E. & Vezina, P. Previous exposure to VTA amphetamine enhances cocaine self-administration under a progressive ratio schedule in an NMDA, AMPA/kainate, and metabotropic glutamate receptor–dependent manner. Neuropsychopharmacology 28, 629–639 (2003).
LaLumiere, R.T. & Kalivas, P.W. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J. Neurosci. 28, 3170–3177 (2008).
Mitrano, D.A. & Smith, Y. Comparative analysis of the subcellular and subsynaptic localization of mGluR1a and mGluR5 metabotropic glutamate receptors in the shell and core of the nucleus accumbens in rat and monkey. J. Comp. Neurol. 500, 788–806 (2007).
Chiamulera, C. et al. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat. Neurosci. 4, 873–874 (2001).
De Roo, M., Klauser, P., Garcia, P.M., Poglia, L. & Muller, D. Spine dynamics and synapse remodeling during LTP and memory processes. Prog. Brain Res. 169, 199–207 (2008).
Lavin, A. et al. Mesocortical dopamine neurons operate in distinct temporal domains using multimodal signaling. J. Neurosci. 25, 5013–5023 (2005).
Goto, Y. & Grace, A.A. Dopamine-dependent interactions between limbic and prefrontal cortical plasticity in the nucleus accumbens: disruption by cocaine sensitization. Neuron 47, 255–266 (2005).
West, A.R., Moore, H. & Grace, A.A. Direct examination of local regulation of membrane activity in striatal and prefrontal cortical neurons in vivo using simultaneous intracellular recording and microdialysis. J. Pharmacol. Exp. Ther. 301, 867–877 (2002).
Acknowledgements
We thank F. Thiels (University of Pittsburgh) for invaluable advice on field recording. This research was supported by US Public Health Service grants DA03906, DA12513, DA015369 and DA024355.
Author information
Authors and Affiliations
Contributions
K.M. was primary in conducting the study. A.P. assisted in conducting the microdialysis experiments. M.M. collected the initial preliminary data indicating that NAC would reverse the cocaine-induced loss of LTP. A.L. provided training and technical advice for the in vivo recordings. M.F.O. and J.T.G. assisted in the behavioral pharmacology experiments. K.M. and P.W.K. developed the experimental design and made the primary contributions in writing the manuscript and preparing the figures.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 (PDF 323 kb)
Rights and permissions
About this article
Cite this article
Moussawi, K., Pacchioni, A., Moran, M. et al. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci 12, 182–189 (2009). https://doi.org/10.1038/nn.2250
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2250
This article is cited by
-
High frequency DBS-like optogenetic stimulation of nucleus accumbens dopamine D2 receptor-containing neurons attenuates cocaine reinstatement in male rats
Neuropsychopharmacology (2023)
-
Astrocytes in cocaine addiction and beyond
Molecular Psychiatry (2022)
-
Astrocytes in the ventral pallidum extinguish heroin seeking through GAT-3 upregulation and morphological plasticity at D1-MSN terminals
Molecular Psychiatry (2022)
-
Mechanistic Effects and Use of N-acetylcysteine in Substance Use Disorders
Current Behavioral Neuroscience Reports (2022)
-
Addiction-induced plasticity in underlying neural circuits
Neurological Sciences (2022)