Abstract
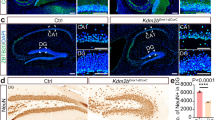
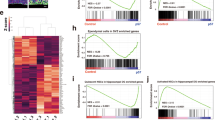
Neural stem cells (NSCs) are controlled by diffusible factors. The transcription factor Sox2 is expressed by NSCs and Sox2 mutations in humans cause defects in the brain and, in particular, in the hippocampus. We deleted Sox2 in the mouse embryonic brain. At birth, the mice showed minor brain defects; shortly afterwards, however, NSCs and neurogenesis were completely lost in the hippocampus, leading to dentate gyrus hypoplasia. Deletion of Sox2 in adult mice also caused hippocampal neurogenesis loss. The hippocampal developmental defect resembles that caused by late sonic hedgehog (Shh) loss. In mutant mice, Shh and Wnt3a were absent from the hippocampal primordium. A SHH pharmacological agonist partially rescued the hippocampal defect. Chromatin immunoprecipitation identified Shh as a Sox2 target. Sox2-deleted NSCs did not express Shh in vitro and were rapidly lost. Their replication was partially rescued by the addition of SHH and was almost fully rescued by conditioned medium from normal cells. Thus, NSCs control their status, at least partly, through Sox2-dependent autocrine mechanisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
References
Avilion, A.A. et al. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 17, 126–140 (2003).
Masui, S. et al. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat. Cell Biol. 9, 625–635 (2007).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Zappone, M.V., Galli, R., Catena, R. & Nicolis, S.K. Sox2 regulatory sequences direct expression of a beta-geo transgene to telencephalic neural stem cells and precursors of the mouse embryo, revealing regionalization of gene expression in CNS stem cells. Development 127, 2367–2382 (2000).
Suh, H. et al. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell 1, 515–528 (2007).
Fantes, J. et al. Mutations in SOX2 cause anophthalmia. Nat. Genet. 33, 461–463 (2003).
Kelberman, D. et al. Mutations within Sox2/SOX2 are associated with abnormalities in the hypothalamo-pituitary-gonadal axis in mice and humans. J. Clin. Invest. 116, 2442–2455 (2006).
Sisodiya, S.M. et al. Role of SOX2 mutations in human hippocampal malformations and epilepsy. Epilepsia 47, 534–542 (2006).
Ferri, A.L., Cavallaro, M., Braida, D., Di Cristofano, A. & Nicolis, S.K. Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development 131, 3805–3819 (2004).
Cavallaro, M., Mariani, J., Lancini, C., Latorre, E. & Nicolis, S.K. Impaired generation of mature neurons by neural stem cells from hypomorphic Sox2 mutants. Development 135, 541–557 (2008).
Medina, D.L. et al. TrkB regulates neocortex formation through the Shc/PLCγ-mediated control of neuronal migration. EMBO J. 23, 3803–3814 (2004).
Merkle, F.T., Tramontin, A.D., Garcia-Verdugo, J.M. & Alvarez-Buylla, A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc. Natl. Acad. Sci. USA 101, 17528–17532 (2004).
Doetsch, F. The glial identity of neural stem cells. Nat. Neurosci. 6, 1127–1134 (2003).
Roelink, H. Hippocampus formation: an intriguing collaboration. Curr. Biol. 10, R279–R281 (2000).
Lee, S.M., Tole, S., Grove, E. & McMahon, A. A local Wnt3a signal is required for development of the mammalian hippocampus. Development 127, 457–467 (2000).
Machold, R. et al. Sonic Hedgehog is required for progenitor cell maintenance in the telencephalic stem cell niches. Neuron 39, 937–950 (2003).
Frank-Kamenetsky, M. et al. Small-molecule modulators of Hedgehog signaling: identification and characterization of Smoothened agonists and antagonists. J. Biol. 1, 10 (2002).
Weber, P., Metzger, D. & Chambon, P. Temporally controlled targeted somatic mutagenesis in the mouse brain. Eur. J. Neurosci. 14, 1777–1783 (2001).
Akagi, K. et al. Cre-mediated somatic site-specific recombination in mice. Nucleic Acids Res. 25, 1766–1773 (1997).
Kempermann, G., Jessberger, S., Steiner, B. & Kronenberg, G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 27, 447–452 (2004).
Gritti, A. et al. Multipotential stem cells from the adult mouse brain proliferate and self-renew in response to basic fibroblast growth factor. J. Neurosci. 16, 1091–1100 (1996).
Miyagi, S. et al. Consequence of the loss of Sox2 in the developing brain of the mouse. FEBS Lett. 582, 2811–2815 (2008).
Lai, K., Kaspar, B.K., Gage, F.H. & Schaffer, D.V. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat. Neurosci. 6, 21–27 (2003).
Palma, V. & Ruiz i Altaba, A. Hedgehog-GLI signaling regulates the behavior of cells with stem cell properties in the developing neocortex. Development 131, 337–345 (2004).
Palma, V. et al. Sonic hedgehog controls stem cells behavior in the postnatal and adult brain. Development 132, 335–344 (2005).
Balordi, F. & Fishell, G. Hedgehog signaling in the subventricular zone is required both for the maintenance of stem cells and the migration of newborn neurons. J. Neurosci. 27, 5936–5947 (2007).
Ahn, S. & Joyner, A.L. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature 437, 894–897 (2005).
Wang, Y., McMahon, A.P. & Allen, B.L. Shifting paradigms in Hedgehog signaling. Curr. Opin. Cell Biol. 19, 159–165 (2007).
Sato, N., Meijer, L., Skaltsounis, L., Greengard, P. & Brivanlou, A.H. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat. Med. 10, 55–63 (2004).
Jeong, Y., El Jaick, K., Roessler, E., Muenke, M. & Epstein, D.J. A functional screen for sonic hedgehog regulatory elements across a 1 Mb interval identifies long-range ventral forebrain enhancers. Development 133, 761–772 (2006).
Taranova, O.V. et al. SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes Dev. 20, 1187–1202 (2006).
Fasano, C.A. et al. shRNA knockdown of Bmi-1 reveals a critical role for p21-Rb pathway in NSC self-renewal during development. Cell Stem Cell 1, 87–99 (2007).
Shi, Y. et al. Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature 427, 78–83 (2004).
Riquelme, P.A., Drapeau, E. & Doetsch, F. Brain micro-ecologies: neural stem cell niches in the adult mammalian brain. Phil. Trans. R. Soc. Lond. B 363, 123–137 (2008).
Gilbertson, R.J. & Rich, J.N. Making a tumour's bed: glioblastoma stem cells and the vascular niche. Nat. Rev. Cancer 7, 733–736 (2007).
Marson, A. et al. Wnt signaling promotes reprogramming of somatic cells to pluripotency. Cell Stem Cell 3, 132–135 (2008).
Lee, J. et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 9, 391 (2006).
Clement, V., Sanchez, P., de Tribolet, N., Radovanovic, I. & Altaba, A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr. Biol. 17, 165–172 (2007).
Schmitz, M. et al. Identification of SOX2 as a novel glioma-associated antigen and potential target for T cell–based immunotherapy. Br. J. Cancer 96, 1293–1301 (2007).
Romer, J.T. et al. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/−)p53(−/−) mice. Cancer Cell 6, 229 (2004).
Farley, F.W., Soriano, P., Steffen, L.S. & Dymecki, S.M. Widespread recombinase expression using FLPeR (flipper) mice. Genesis 28, 106–110 (2000).
Nyfeler, Y. et al. Jagged1 signals in the postnatal subventricular zone are required for neural stem cell self-renewal. EMBO J. 24, 3504–3515 (2005).
Conlon, R.A. & Herrmann, B.G. Detection of messenger RNA by in situ hybridization to postimplantation embryo whole mounts. Methods Enzymol. 225, 373–383 (1993).
Echelard, Y. et al. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 75, 1417–1430 (1993).
Weinmann, A.S. & Farnham, P.J. Identification of unknown target genes of human transcription factors using chromatin immunoprecipitation. Methods 26, 37–47 (2002).
Szutorisz, H. et al. Formation of an active tissue-specific chromatin domain initiated by epigenetic marking at the embryonic stem cell stage. Mol. Cell. Biol. 25, 1804–1820 (2005).
Acknowledgements
We thank G. Dehò and S. Zangrossi for the pcnB∷Tn10 recA∷cat C-5507 E. coli C strain, S. Dymecki for the pLM-FLRT-3 vector and the FLPeR mouse strain, R. Klein through Jackson Laboratories for the nestin-cre mouse strain, P. Chambon for the creERT2 gene, Curis for the 1.2 SHH agonist18, S. Lugert for help with the Notch pathway expression studies, J. Cozzi and GenOway for help with ES cell targeting, C. Tiveron and Layline Genomics for blastocyst injections, C. Tiveron and L. Tatangelo for Sox2-creERT2 pronuclear injections, R. Caccia for help with in situ hybridization, E. Bagnaresi and A. Marinelli for help with constructs, A. Ronchi for advice on EMSAs and ChIP and D. Santoni for animal care. The monoclonal antibody to SHH developed by T.M. Jessell was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the US National Institute of Child Health and Human Development and maintained by the University of Iowa. This work was supported by Telethon (GGP05122), the European Economic Community (STEMBRIDGE), Fondazione Cariplo, Associazione Italiana Ricerca sul Cancro, Fondazione Banca del Monte di Lombardia, the Ministero Istruzione Università e Ricerca (Cofin) and FAR 2004-7 grants to S.K.N., and by the Max Planck Gesellschaft through V. Taylor.
Author information
Authors and Affiliations
Contributions
R.F. and M.V. generated the Sox2 mutations in ES cells, and carried out animal breeding, cell cultures and RT-PCR. A.L.M.F. performed in situ hybridization and immunohistochemistry. E.L. carried out ChIP and EMSAs. J.M. performed the lentiviral rescue experiment. C.G. carried out the Notch signaling studies and initial work with in vitro cultures and for in vitro SOX2 deletion. C.L. participated in immunochemical studies. V. Tosetti participated in the in vitro culture work and immunocytochemistry. S.O. discussed the experiments and wrote the paper. V. Taylor participated in the in vivo rescue experiments, discussed data and supervised initial in vitro studies. S.K.N. devised the experiments, supervised the work and wrote the paper.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 (PDF 2372 kb)
Rights and permissions
About this article
Cite this article
Favaro, R., Valotta, M., Ferri, A. et al. Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat Neurosci 12, 1248–1256 (2009). https://doi.org/10.1038/nn.2397
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2397
This article is cited by
-
Inhibition of TGF-β signaling enables long-term proliferation of mouse primary epithelial stem/progenitor cells of the tympanic membrane and the middle ear mucosa
Scientific Reports (2023)
-
Enhancer–promoter interactions can bypass CTCF-mediated boundaries and contribute to phenotypic robustness
Nature Genetics (2023)
-
The rates of adult neurogenesis and oligodendrogenesis are linked to cell cycle regulation through p27-dependent gene repression of SOX2
Cellular and Molecular Life Sciences (2023)
-
Drugs and Endogenous Factors as Protagonists in Neurogenic Stimulation
Stem Cell Reviews and Reports (2022)
-
Role of Sonic Hedgehog Signaling Activation in the Prevention of Neurological Abnormalities Associated with Obsessive–Compulsive Disorder
Neurotoxicity Research (2022)