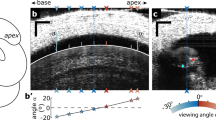
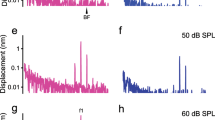
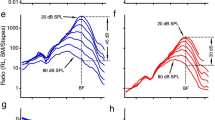
Abstract
The ear is a remarkably sensitive pressure fluctuation detector. In guinea pigs, behavioral measurements indicate a minimum detectable sound pressure of ∼20 μPa at 16 kHz. Such faint sounds produce 0.1-nm basilar membrane displacements, a distance smaller than conformational transitions in ion channels. It seems that noise within the auditory system would swamp such tiny motions, making weak sounds imperceptible. Here we propose a new mechanism contributing to a resolution of this problem and validate it through direct measurement. We hypothesized that vibration at the apical side of hair cells is enhanced compared with that at the commonly measured basilar membrane side. Using in vivo optical coherence tomography, we demonstrated that apical-side vibrations peaked at a higher frequency, had different timing and were enhanced compared with those at the basilar membrane. These effects depend nonlinearly on the stimulus sound pressure level. The timing difference and enhancement of vibrations are important for explaining how the noise problem is circumvented.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Heffner, R., Heffner, H. & Masterton, B. Behavioral measurements of absolute and frequency-difference thresholds in guinea pig. J. Acoust. Soc. Am. 49, 1888–1895 (1971).
Prosen, C.A., Petersen, M.R., Moody, D.B. & Stebbins, W.C. Auditory thresholds and kanamycin-induced hearing loss in the guinea pig assessed by a positive reinforcement procedure. J. Acoust. Soc. Am. 63, 559–566 (1978).
Hood, L.J. et al. Objective auditory threshold estimation using sine-wave derived responses. Hear. Res. 55, 109–116 (1991).
Cooper, N.P. & Guinan, J.J. Jr. Efferent-mediated control of basilar membrane motion. J. Physiol. (Lond.) 576, 49–54 (2006).
Khanna, S.M. & Leonard, D.G.B. Basilar membrane tuning in the cat cochlea. Science 215, 305–306 (1982).
Sellick, P.M., Patuzzi, R. & Johnstone, B.M. Measurement of basilar membrane motion in the guinea pig using Mössbauer technique. J. Acoust. Soc. Am. 72, 131–141 (1982).
Fridberger, A., Tomo, I., Ulfendahl, M. & Boutet de Monvel, J. Imaging hair cell transduction at the speed of sound: dynamic behavior of mammalian stereocilia. Proc. Natl. Acad. Sci. USA 103, 1918–1923 (2006).
Hu, X., Evans, B.N. & Dallos, P. Direct visualization of organ of Corti kinematics in a hemicochlea. J. Neurophysiol. 82, 2798–2807 (1999).
van Netten, S.M., Dinklo, T., Marcotti, W. & Kros, C.J. Channel gating forces govern accuracy of mechano-electrical transduction in hair cells. Proc. Natl. Acad. Sci. USA 100, 15510–15515 (2003).
Denk, W. & Webb, W.W. Thermal-noise-limited transduction observed in mechanosensory receptors of the inner ear. Phys. Rev. Lett. 63, 207–210 (1989).
Bialek, W. Physical limits to sensation and perception. Annu. Rev. Biophys. Biophys. Chem. 16, 455–478 (1987).
Pickles, J.O. An Introduction to the Physiology of Hearing 2nd edn. (Emerald Group, West Yorkshire, 1988).
Barral, J., Dierkes, K., Lindner, B., Jülicher, F. & Martin, P. Coupling a sensory hair-cell bundle to cyber clones enhances nonlinear amplification. Proc. Natl. Acad. Sci. USA 107, 8079–8084 (2010).
Robles, L. & Ruggero, M.A. Mechanics of the mammalian cochlea. Physiol. Rev. 81, 1305–1352 (2001).
Kolston, P.J. Comparing in vitro, in situ, and in vivo experimental data in a three-dimensional model of mammalian cochlear mechanics. Proc. Natl Acad. Sci. USA 96, 3676–3681 (1999).
Olson, E.S. & Mountain, D.C. In vivo measurement of basilar membrane stiffness. J. Acoust. Soc. Am. 89, 1262–1275 (1991).
Flock, Å. Transducing mechanisms in the lateral line canal organ receptors. Cold Spring Harb. Symp. Quant. Biol. 30, 133–145 (1965).
Hudspeth, A.J. & Corey, D.P. Sensitivity, polarity, and conductance change in the response of vertebrate hair cells to controlled mechanical stimuli. Proc. Natl. Acad. Sci. USA 74, 2407–2411 (1977).
Corey, D.P. & Hudspeth, A.J. Ionic basis of the receptor potential in a vertebrate hair cell. Nature 281, 675–677 (1979).
Mammano, F. & Ashmore, J.F. Reverse transduction measured in the isolated cochlea by laser Michelson interferometry. Nature 365, 838–841 (1993).
Choudhury, N. et al. Low coherence interferometry of the cochlear partition. Hear. Res. 220, 1–9 (2006).
Chen, F. et al. In vivo imaging and low-coherence interferometry of organ of Corti vibration. J. Biomed. Opt. 12, 021006 (2007).
Rhode, W.S. Observations of the vibration of the basilar membrane in squirrel monkeys using the Mössbauer technique. J. Acoust. Soc. Am. 49 (suppl. 2): 1218–1231 (1971).
Nuttall, A.L., Dolan, D.F. & Avinash, G. Laser Doppler velocimetry of basilar membrane vibration. Hear. Res. 51, 203–213 (1991).
Fridberger, A. et al. Organ of Corti potentials and the motion of the basilar membrane. J. Neurosci. 24, 10057–10063 (2004).
Brownell, W.E., Bader, C.R., Bertrand, D. & de Ribaupierre, Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science 227, 194–196 (1985).
Frank, G., Hemmert, W. & Gummer, A.W. Limiting dynamics of high-frequency electromechanical transduction of outer hair cells. Proc. Natl. Acad. Sci. USA 96, 4420–4425 (1999).
Nuttall, A.L. & Ren, T. Electromotile hearing: evidence from basilar membrane motion and otoacoustic emissions. Hear. Res. 92, 170–177 (1995).
Zheng, J. et al. Prestin is the motor protein of cochlear outer hair cells. Nature 405, 149–155 (2000).
Chan, D.K. & Hudspeth, A.J. Ca2+ current-driven nonlinear amplification by the mammalian cochlea in vitro. Nat. Neurosci. 8, 149–155 (2005).
Kennedy, H.J., Crawford, A.C. & Fettiplace, R. Force generation by mammalian hair bundles supports a role in cochlear amplification. Nature 433, 880–883 (2005).
Denk, W. & Webb, W.W. Forward and reverse transduction at the limit of sensitivity studied by correlating electrical and mechanical fluctuations in frog saccular hair cells. Hear. Res. 60, 89–102 (1992).
Jaramillo, F. & Wiesenfeld, K. Mechanoelectrical transduction assisted by Brownian motion: a role for noise in the auditory system. Nat. Neurosci. 1, 384–388 (1998).
Ruggero, M.A. & Temchin, A.N. Unexceptional sharpness of frequency tuning in the human cochlea. Proc. Natl. Acad. Sci. USA 102, 18614–18619 (2005).
de Boer, E. & Nuttall, A.L. The mechanical waveform of the basilar membrane. III. Intensity effects. J. Acoust. Soc. Am. 107, 1497–1507 (2000).
Allen, J.B. Cochlear micromechanics—a physical model of tranduction. J. Acoust. Soc. Am. 68, 1660–1670 (1980).
Gummer, A.W., Hemmert, W. & Zenner, H.-P. Resonant tectorial membrane motion in the inner ear: its crucial role in frequency tuning. Proc. Natl. Acad. Sci. USA 93, 8727–8732 (1996).
Zwislocki, J.J. & Kletsky, E.J. Tectorial membrane: a possible effect on frequency analysis in the cochlea. Science 204, 639–641 (1979).
Dallos, P. in Biophysics of the Cochlea: Molecules to Models (ed. Gummer, A.W.) 97–109 (World Scientific, Titisee, Germany, 2002).
Ruggero, M.A. & Rich, N.C. Furosemide alters organ of corti mechanics: evidence for feedback of outer hair cells upon the basilar membrane. J. Neurosci. 11, 1057–1067 (1991).
Narayan, S.S., Temchin, A.N., Recio, A. & Ruggero, M.A. Frequency tuning of basilar membrane and auditory nerve fibers in the same cochleae. Science 282, 1882–1884 (1998).
Fridberger, A. & de Monvel, J.B. Sound-induced differential motion within the hearing organ. Nat. Neurosci. 6, 446–448 (2003).
Tomo, I., Boutet de Monvel, J. & Fridberger, A. Sound-evoked radial strain in the hearing organ. Biophys. J. 93, 3279–3284 (2007).
Khanna, S.M. & Hao, L.F. Amplification in the apical turn of the cochlea with negative feedback. Hear. Res. 149, 55–76 (2000).
Baden-Kristensen, K. & Weiss, T.F. Receptor potentials of lizard hair cells with free-standing stereocilia: responses to acoustic clicks. J. Physiol. 335, 699–721 (1983).
Acknowledgements
We thank E. de Boer, T. Ren, A. Magnusson and P. Gillespie for critical discussions and reading of the manuscript. This work was supported by US National Institutes of Health, National Institute on Deafness and Other Communication Disorders grants DC00141 (A.L.N.), DC010399 (A.L.N.) and DC010201 (R.K.W.); and Swedish Research Council grant K2008-63X-14061-08-3, the Tysta Skolan Foundation and Hörselskadades Riksförbund (A.F.).
Author information
Authors and Affiliations
Contributions
F.C., D.Z., A.F., J.Z. and N.C. conducted experiments. F.C., D.Z., A.F., J.Z., X.S. and A.L.N. analyzed and interpreted data. F.C., A.F., J.Z. and A.L.N. wrote the manuscript. F.C., N.C., S.L.J. and R.K.W. designed and built the OCT interferometer. F.C., A.F., J.Z. and A.L.N. designed the experiments. F.C., D.Z., A.F. and J.Z. contributed equally.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Chen, F., Zha, D., Fridberger, A. et al. A differentially amplified motion in the ear for near-threshold sound detection. Nat Neurosci 14, 770–774 (2011). https://doi.org/10.1038/nn.2827
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2827