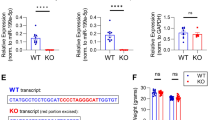
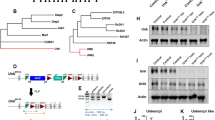
Abstract
A major goal of biomedical research is the identification of molecular and cellular mechanisms that underlie memory storage. Here we report a previously unknown signaling pathway that is necessary for the conversion from short- to long-term memory. The mammalian target of rapamycin (mTOR) complex 2 (mTORC2), which contains the regulatory protein Rictor (rapamycin-insensitive companion of mTOR), was discovered only recently and little is known about its function. We found that conditional deletion of Rictor in the postnatal murine forebrain greatly reduced mTORC2 activity and selectively impaired both long-term memory (LTM) and the late phase of hippocampal long-term potentiation (L-LTP). We also found a comparable impairment of LTM in dTORC2-deficient flies, highlighting the evolutionary conservation of this pathway. Actin polymerization was reduced in the hippocampus of mTORC2-deficient mice and its restoration rescued both L-LTP and LTM. Moreover, a compound that promoted mTORC2 activity converted early LTP into late LTP and enhanced LTM. Thus, mTORC2 could be a therapeutic target for the treatment of cognitive dysfunction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
10 March 2013
In the version of this article initially published online, acknowledgment of grant BCM IDDRC 5P30HD024064-23 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development to M.C.-M. was missing. The error has been corrected for the print, PDF and HTML versions of this article.
10 March 2013
In the version of this supplementary file originally posted online, in Supplementary Figure 11, the wrong images were provided for total S6K for Fig. 1a, β-actin for Figures 2a and 3a, actin (bottom) for Fig. 5a and p-Akt for Figure 6a, and the panels corresponding to Figures 6a, 6b, 6d and 6e were mislabeled 5b, 5c, 5d and 5e, respectively. The errors have been corrected in this file as of 10 March 2013.
References
Kandel, E.R. The molecular biology of memory storage: a dialogue between genes and synapses. Science 294, 1030–1038 (2001).
McGaugh, J.L. Memory–a century of consolidation. Science 287, 248–251 (2000).
Costa-Mattioli, M., Sossin, W.S., Klann, E. & Sonenberg, N. Translational control of long-lasting synaptic plasticity and memory. Neuron 61, 10–26 (2009).
Wang, S.H. & Morris, R.G. Hippocampal-neocortical interactions in memory formation, consolidation and reconsolidation. Annu. Rev. Psychol. 61, 49–79 C1–4 (2010).
Cingolani, L.A. & Goda, Y. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat. Rev. Neurosci. 9, 344–356 (2008).
Lamprecht, R. & LeDoux, J. Structural plasticity and memory. Nat. Rev. Neurosci. 5, 45–54 (2004).
Lynch, G., Rex, C.S. & Gall, C.M. LTP consolidation: substrates, explanatory power and functional significance. Neuropharmacology 52, 12–23 (2007).
Guertin, D.A. & Sabatini, D.M. Defining the role of mTOR in cancer. Cancer Cell 12, 9–22 (2007).
Wullschleger, S., Loewith, R. & Hall, M.N. TOR signaling in growth and metabolism. Cell 124, 471–484 (2006).
Ma, X.M. & Blenis, J. Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 10, 307–318 (2009).
Oh, W.J. & Jacinto, E. mTOR complex 2 signaling and functions. Cell Cycle 10, 2305–2316 (2011).
Guertin, D.A. et al. Ablation in mice of the mTORC components raptor, rictor or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev. Cell 11, 859–871 (2006).
Shiota, C., Woo, J.T., Lindner, J., Shelton, K.D. & Magnuson, M.A. Multiallelic disruption of the rictor gene in mice reveals that mTOR complex 2 is essential for fetal growth and viability. Dev. Cell 11, 583–589 (2006).
Carson, R.P., Fu, C., Winzenburger, P. & Ess, K.C. Deletion of Rictor in neural progenitor cells reveals contributions of mTORC2 signaling to tuberous sclerosis complex. Hum. Mol. Genet. 22, 140–152 (2013).
Siuta, M.A. et al. Dysregulation of the norepinephrine transporter sustains cortical hypodopaminergia and schizophrenia-like behaviors in neuronal rictor null mice. PLoS Biol. 8, e1000393 (2010).
Mazei-Robison, M.S. et al. Role for mTOR signaling and neuronal activity in morphine-induced adaptations in ventral tegmental area dopamine neurons. Neuron 72, 977–990 (2011).
Griffin, R.J. et al. Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer's disease pathology. J. Neurochem. 93, 105–117 (2005).
Humbert, S. et al. The IGF-1/Akt pathway is neuroprotective in Huntington's disease and involves Huntingtin phosphorylation by Akt. Dev. Cell 2, 831–837 (2002).
Malagelada, C., Jin, Z.H. & Greene, L.A. RTP801 is induced in Parkinson's disease and mediates neuron death by inhibiting Akt phosphorylation/activation. J. Neurosci. 28, 14363–14371 (2008).
Meikle, L. et al. Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J. Neurosci. 28, 5422–5432 (2008).
Siarey, R.J. et al. Altered signaling pathways underlying abnormal hippocampal synaptic plasticity in the Ts65Dn mouse model of Down syndrome. J. Neurochem. 98, 1266–1277 (2006).
Dragatsis, I. & Zeitlin, S. CaMKIIalpha-Cre transgene expression and recombination patterns in the mouse brain. Genesis 26, 133–135 (2000).
Tsien, J.Z. et al. Subregion- and cell type–restricted gene knockout in mouse brain. Cell 87, 1317–1326 (1996).
LeDoux, J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 23, 155–184 (2000).
Morris, R.G., Garrud, P., Rawlins, J.N. & O'Keefe, J. Place navigation impaired in rats with hippocampal lesions. Nature 297, 681–683 (1982).
Hietakangas, V. & Cohen, S.M. Re-evaluating AKT regulation: role of TOR complex 2 in tissue growth. Genes Dev. 21, 632–637 (2007).
Sarbassov, D.D., Guertin, D.A., Ali, S.M. & Sabatini, D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307, 1098–1101 (2005).
Tully, T., Preat, T., Boynton, S.C. & Del Vecchio, M. Genetic dissection of consolidated memory in Drosophila. Cell 79, 35–47 (1994).
Heasman, S.J. & Ridley, A.J. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat. Rev. Mol. Cell Biol. 9, 690–701 (2008).
Tolias, K.F. et al. The Rac1-GEF Tiam1 couples the NMDA receptor to the activity-dependent development of dendritic arbors and spines. Neuron 45, 525–538 (2005).
Holzinger, A. Jasplakinolide: an actin-specific reagent that promotes actin polymerization. Methods Mol. Biol. 586, 71–87 (2009).
Han, E.K. et al. Akt inhibitor A-443654 induces rapid Akt Ser-473 phosphorylation independent of mTORC1 inhibition. Oncogene 26, 5655–5661 (2007).
Kopec, C.D., Li, B., Wei, W., Boehm, J. & Malinow, R. Glutamate receptor exocytosis and spine enlargement during chemically induced long-term potentiation. J. Neurosci. 26, 2000–2009 (2006).
Fukazawa, Y. et al. Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron 38, 447–460 (2003).
Matsuzaki, M., Honkura, N., Ellis-Davies, G.C. & Kasai, H. Structural basis of long-term potentiation in single dendritic spines. Nature 429, 761–766 (2004).
Okamoto, K., Nagai, T., Miyawaki, A. & Hayashi, Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat. Neurosci. 7, 1104–1112 (2004).
Chen, L.Y., Rex, C.S., Casale, M.S., Gall, C.M. & Lynch, G. Changes in synaptic morphology accompany actin signaling during LTP. J. Neurosci. 27, 5363–5372 (2007).
Kim, C.H. & Lisman, J.E. A role of actin filament in synaptic transmission and long-term potentiation. J. Neurosci. 19, 4314–4324 (1999).
Kramár, E.A., Lin, B., Rex, C.S., Gall, C.M. & Lynch, G. Integrin-driven actin polymerization consolidates long-term potentiation. Proc. Natl. Acad. Sci. USA 103, 5579–5584 (2006).
Krucker, T., Siggins, G.R. & Halpain, S. Dynamic actin filaments are required for stable long-term potentiation (LTP) in area CA1 of the hippocampus. Proc. Natl. Acad. Sci. USA 97, 6856–6861 (2000).
Fischer, A., Sananbenesi, F., Schrick, C., Spiess, J. & Radulovic, J. Distinct roles of hippocampal de novo protein synthesis and actin rearrangement in extinction of contextual fear. J. Neurosci. 24, 1962–1966 (2004).
Lamprecht, R., Farb, C.R. & LeDoux, J.E. Fear memory formation involves p190 RhoGAP and ROCK proteins through a GRB2-mediated complex. Neuron 36, 727–738 (2002).
Mantzur, L., Joels, G. & Lamprecht, R. Actin polymerization in lateral amygdala is essential for fear memory formation. Neurobiol. Learn. Mem. 91, 85–88 (2009).
Bourtchuladze, R. et al. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element–binding protein. Cell 79, 59–68 (1994).
Costa-Mattioli, M. et al. eIF2alpha phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell 129, 195–206 (2007).
Olson, E.N. & Nordheim, A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat. Rev. Mol. Cell Biol. 11, 353–365 (2010).
Van Horck, F.P. & Holt, C.E. A cytoskeletal platform for local translation in axons. Sci. Signal. 1, pe11 (2008).
Choe, G. et al. Analysis of the phosphatidylinositol 3′-kinase signaling pathway in glioblastoma patients in vivo. Cancer Res. 63, 2742–2746 (2003).
Tsai, V. et al. Fetal brain mTOR signaling activation in tuberous sclerosis complex. Cereb. Cortex published online, doi:10.1093/cercor/bhs310 (18 October 2012).
Zhou, J. et al. Pharmacological inhibition of mTORC1 suppresses anatomical, cellular and behavioral abnormalities in neural-specific Pten knock-out mice. J. Neurosci. 29, 1773–1783 (2009).
Stoica, L. et al. Selective pharmacogenetic inhibition of mammalian target of Rapamycin complex I (mTORC1) blocks long-term synaptic plasticity and memory storage. Proc. Natl. Acad. Sci. USA 108, 3791–3796 (2011).
Zhu, P.J. et al. Suppression of PKR promotes network excitability and enhanced cognition by interferon gamma–mediated disinhibition. Cell 147, 1384–1396 (2011).
Shamah, S.M. et al. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell 105, 233–244 (2001).
Zeng, L.H. et al. Kainate seizures cause acute dendritic injury and actin depolymerization in vivo. J. Neurosci. 27, 11604–11613 (2007).
Ferris, J., Ge, H., Liu, L. & Roman, G. G(o) signaling is required for Drosophila associative learning. Nat. Neurosci. 9, 1036–1040 (2006).
Acknowledgements
We thank M. Magnuson (Vanderbilt University), I. Dragatsis (University of Tennessee) and K. Tolias (Baylor College of Medicine) who generously provided RictorloxP/loxP mice, Camk2a-Cre mice and the Tiam1 constructs, respectively. We also thank A. Placzek and W. Sossin for comments on an early version of the manuscript. This work was supported by grants to M.C.-M. (National Institute of Mental Health grant MH 096816, National Institute of Neurological Disorders and Stroke grant NS 076708, Searle award grant 09-SSP-211, Whitehall award, grants from the George and Cynthia Mitchell Foundation, and Eunice Kennedy Shriver National Institute of Child Health & Human Development BCM IDDRC 5P30HD024064-23) and G.R. (US National Institutes of Health grant MH091305 and a Texas Norman Hackerman Advanced Research Program award).
Author information
Authors and Affiliations
Contributions
W.H., P.J.Z. and M.C.-M. conceived and designed the experiments. W.H. performed the behavioral, molecular and spine density experiments. P.J.Z. performed the electrophysiology experiments. H.Z. performed the Nissl staining experiments. G.R. and S.Z. performed and analyzed the Drosophila behavioral experiments. All of the authors discussed the results and commented on the manuscript. M.C.-M. wrote the manuscript with input from W.H., G.R., P.J.Z. and K.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 (PDF 4456 kb)
Rights and permissions
About this article
Cite this article
Huang, W., Zhu, P., Zhang, S. et al. mTORC2 controls actin polymerization required for consolidation of long-term memory. Nat Neurosci 16, 441–448 (2013). https://doi.org/10.1038/nn.3351
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3351
This article is cited by
-
mTOR hypoactivity leads to trophectoderm cell failure by enhancing lysosomal activation and disrupting the cytoskeleton in preimplantation embryo
Cell & Bioscience (2023)
-
LCRMP-1 is required for spermatogenesis and stabilises spermatid F-actin organization via the PI3K-Akt pathway
Communications Biology (2023)
-
Targeted suppression of mTORC2 reduces seizures across models of epilepsy
Nature Communications (2023)
-
Systemic changes in cell size throughout the body of Drosophila melanogaster associated with mutations in molecular cell cycle regulators
Scientific Reports (2023)
-
Septin11 promotes hepatocellular carcinoma cell motility by activating RhoA to regulate cytoskeleton and cell adhesion
Cell Death & Disease (2023)