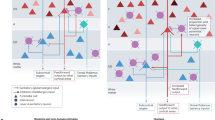
Abstract
All the higher mental and cognitive functions unique to humans depend on the neocortex ('new' cortex, referring to its relatively recent appearance in evolution), which is divided into discrete areas that subserve distinct functions, such as language, movement and sensation. With a few notable exceptions, all neocortical areas have six layers of neurons and a remarkably similar thickness and overall cell density, despite subtle differences in their cellular architecture. Furthermore, all neocortical areas are formed over roughly the same time period during development and provide little hint at early developmental stages of the rich functional diversity that becomes apparent as development comes to an end. How these areas are formed has long fascinated developmental neuroscientists, because the formation of new cortical areas, with the attendant appearance of new cortical functions, is what must have driven the evolution of mammalian behavior.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Amy Center





Similar content being viewed by others
References
Van der Loos, H. & Woolsey, T. A. Somatosensory cortex: structural alterations following early injury to sense organs. Science 179, 395–398 (1973).
O'Leary, D. D. Do cortical areas emerge from a protocortex? Trends Neurosci. 12, 400–406 (1989).
Sur, M. & Leamey, C. A. Development and plasticity of cortical areas and networks. Nat. Rev. Neurosci. 2, 251–262 (2001).
Levitt, P. A monoclonal antibody to limbic system neurons. Science 223, 299–301 (1984).
Barbe, M. F. & Levitt, P. The early commitment of fetal neurons to the limbic cortex. J. Neurosci. 11, 519–533 (1991).
Arimatsu, Y. et al. Early regional specification for a molecular neuronal phenotype in the rat neocortex. Proc. Natl. Acad. Sci. USA 89, 8879–8883 (1992).
Cohen-Tannoudji, M., Babinet, C. & Wassef, M. Early determination of a mouse somatosensory cortex marker. Nature 368, 460–463 (1994).
Miyashita-Lin, E. M., Hevner, R., Wassarman, K. M., Martinez, S. & Rubenstein, J. L. Early neocortical regionalization in the absence of thalamic innervation. Science 285, 906–909 (1999).
Nakagawa, Y., Johnson, J. E. & O'Leary, D. D. Graded and areal expression patterns of regulatory genes and cadherins in embryonic neocortex independent of thalamocortical input. J. Neurosci. 19, 10877–10885 (1999).
Rakic, P. Specification of cerebral cortical areas. Science 241, 170–176 (1988).
Wilson, S. W. & Rubenstein, J. L. Induction and dorsoventral patterning of the telencephalon. Neuron 28, 641–651 (2000).
Furuta, Y., Piston, D. W. & Hogan, B. L. Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development 124, 2203–2212 (1997).
Mione, M. C., Danevic, C., Boardman, P., Harris, B. & Parnavelas, J. G. Lineage analysis reveals neurotransmitter (GABA or glutamate) but not calcium-binding protein homogeneity in clonally related cortical neurons. J. Neurosci. 14, 107–123 (1994).
Tan, S. S. et al. Separate progenitors for radial and tangential cell dispersion during development of the cerebral neocortex. Neuron 21, 295–304 (1998).
Ware, M. L., Tavazoie, S. F., Reid, C. B. & Walsh, C. A. Coexistence of widespread clones and large radial clones in early embryonic ferret cortex. Cereb. Cortex 9, 636–645 (1999).
Parnavelas, J. G. The origin and migration of cortical neurones: new vistas. Trends Neurosci. 23, 126–131 (2000).
Noctor, S. C., Flint, A. C., Weissman, T. A., Dammerman, R. S. & Kriegstein, A. R. Neurons derived from radial glial cells establish radial units in neocortex. Nature 409, 714–720 (2001).
Spreafico, R. et al. Cortical dysplasia: an immunocytochemical study of three patients. Neurology 50, 27–36 (1998).
Najm, I. M. et al. Epileptogenicity correlated with increased N-methyl-D-aspartate receptor subunit NR2A/B in human focal cortical dysplasia. Epilepsia 41, 971–976 (2000).
Crino, P. B., Duhaime, A. C., Baltuch, G. & White, R. Differential expression of glutamate and GABA-A receptor subunit mRNA in cortical dysplasia. Neurology 56, 906–913 (2001).
Walsh, C. & Cepko, C. L. Widespread dispersion of neuronal clones across functional regions of the cerebral cortex. Science 255, 434–440 (1992).
Reid, C. B., Liang, I. & Walsh, C. Systematic widespread clonal organization in cerebral cortex. Neuron 15, 299–310 (1995).
McCarthy, M., Turnbull, D. H., Walsh, C. A. & Fishell, G. Telencephalic neural progenitors appear to be restricted to regional and glial fates before the onset of neurogenesis. J. Neurosci. 21, 6772–6781 (2001).
Anderson, S. A., Eisenstat, D. D., Shi, L. & Rubenstein, J. L. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science 278, 474–476 (1997).
Lavdas, A. A., Grigoriou, M., Pachnis, V. & Parnavelas, J. G. The medial ganglionic eminence gives rise to a population of early neurons in the developing cerebral cortex. J. Neurosci. 19, 7881–7888 (1999).
Anderson, S., Mione, M., Yun, K. & Rubenstein, J. L. Differential origins of neocortical projection and local circuit neurons: role of Dlx genes in neocortical interneuronogenesis. Cereb. Cortex 9, 646–654 (1999).
Reid, C. B., Tavazoie, S. F. & Walsh, C. A. Clonal dispersion and evidence for asymmetric cell division in ferret cortex. Development 124, 2441–2450 (1997).
Monuki, E. S., Porter, F. D. & Walsh, C. A. Patterning of the dorsal telencephalon and cerebral cortex by a roof plate-Lhx2 pathway. Neuron (in press).
Keverne, E. B. GABA-ergic neurons and the neurobiology of schizophrenia and other psychoses. Brain Res. Bull. 48, 467–473 (1999).
Benes, F. M. & Berretta, S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology 25, 1–27 (2001).
Shimamura, K. & Rubenstein, J. L. Inductive interactions direct early regionalization of the mouse forebrain. Development 124, 2709–2718 (1997).
Tao, W. & Lai, E. Telencephalon-restricted expression of BF-1, a new member of the HNF-3/fork head gene family, in the developing rat brain. Neuron 8, 957–966 (1992).
Xuan, S. et al. Winged helix transcription factor BF-1 is essential for the development of the cerebral hemispheres. Neuron 14, 1141–1152 (1995).
Dou, C. L., Li, S. & Lai, E. Dual role of brain factor-1 in regulating growth and patterning of the cerebral hemispheres. Cereb. Cortex 9, 543–550 (1999).
Golden, J. A. Holoprosencephaly: a defect in brain patterning. J. Neuropathol. Exp. Neurol. 57, 991–999 (1998).
Yakovlev, P. I. Pathoarchitectonic studies of cerebral malformations: III. Arrhinencephalies (Holoprosencephaly). J. Neuropathol. Exp. Neurol. 18, 22–55 (1959).
Muenke, M. & Beachy, P. A. Genetics of ventral forebrain development and holoprosencephaly. Curr. Opin. Genet. Dev. 10, 262–269 (2000).
Chiang, C. et al. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383, 407–413 (1996).
Jessell, T. M. & Lumsden, A. in Molecular and Cellular Approaches to Neural Development (eds. Cowan, W. M., Jessell, T. M. & Zipursky, S. L.) 290–333 (Oxford Univ. Press, New York, 1997).
Sussel, L., Marin, O., Kimura, S. & Rubenstein, J. L. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development 126, 3359–3370 (1999).
Wallis, D. E. et al. Mutations in the homeodomain of the human SIX3 gene cause holoprosencephaly. Nat. Genet. 22, 196–198 (1999).
Pasquier, L. et al. A new mutation in the six-domain of SIX3 gene causes holoprosencephaly. Eur. J. Hum. Genet. 8, 797–800 (2000).
Brown, S. A. et al. Holoprosencephaly due to mutations in ZIC2, a homologue of Drosophila odd-paired. Nat. Genet. 20, 180–183 (1998).
Brown, L. Y. et al. Holoprosencephaly due to mutations in ZIC2: alanine tract expansion mutations may be caused by parental somatic recombination. Hum. Mol. Genet. 10, 791–796 (2001).
Nanni, L. et al. The mutational spectrum of the sonic hedgehog gene in holoprosencephaly: SHH mutations cause a significant proportion of autosomal dominant holoprosencephaly. Hum. Mol. Genet. 8, 2479–2488 (1999).
Gripp, K. W. et al. Mutations in TGIF cause holoprosencephaly and link NODAL signalling to human neural axis determination. Nat. Genet. 25, 205–208 (2000).
Oliver, G. et al. Six3, a murine homologue of the sine oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development 121, 4045–4055 (1995).
Nagai, T. et al. The expression of the mouse Zic1, Zic2, and Zic3 gene suggests an essential role for Zic genes in body pattern formation. Dev. Biol. 182, 299–313 (1997).
Nagai, T. et al. Zic2 regulates the kinetics of neurulation. Proc. Natl. Acad. Sci. USA 97, 1618–1623 (2000).
Heldin, C. H., Miyazono, K. & ten Dijke, P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature 390, 465–471 (1997).
Baker, J. C. & Harland, R. M. From receptor to nucleus: the Smad pathway. Curr. Opin. Genet. Dev. 7, 467–473 (1997).
Wotton, D., Lo, R. S., Lee, S. & Massague, J. A Smad transcriptional corepressor. Cell 97, 29–39 (1999).
Fode, C. et al. A role for neural determination genes in specifying the dorsoventral identity of telencephalic neurons. Genes Dev. 14, 67–80 (2000).
Tole, S., Ragsdale, C. W. & Grove, E. A. Dorsoventral patterning of the telencephalon is disrupted in the mouse mutant extra-toes(J). Dev. Biol. 217, 254–265 (2000).
Bulchand, S., Grove, E. A., Porter, F. D. & Tole, S. LIM-homeodomain gene Lhx2 regulates the formation of the cortical hem. Mech. Dev. 100, 165–175 (2001).
Diaz-Benjumea, F. J. & Cohen, S. M. Interaction between dorsal and ventral cells in the imaginal disc directs wing development in Drosophila. Cell 75, 741–752 (1993).
Williams, J. A., Paddock, S. W. & Carroll, S. B. Pattern formation in a secondary field: a hierarchy of regulatory genes subdivides the developing Drosophila wing disc into discrete subregions. Development 117, 571–584 (1993).
Blair, S. S., Brower, D. L., Thomas, J. B. & Zavortink, M. The role of apterous in the control of dorsoventral compartmentalization and PS integrin gene expression in the developing wing of Drosophila. Development 120, 1805–1815 (1994).
Barbe, M. F. & Levitt, P. Attraction of specific thalamic input by cerebral grafts depends on the molecular identity of the implant. Proc. Natl. Acad. Sci. USA 89, 3706–3710 (1992).
Barbe, M. F. & Levitt, P. Age-dependent specification of the corticocortical connections of cerebral grafts. J. Neurosci. 15, 1819–1834 (1995).
Levitt, P. & Eagleson, K. L. Regionalization of the cerebral cortex: developmental mechanisms and models. Novartis Found. Symp. 228, 173–181 (2000).
Lee, K. J. & Jessell, T. M. The specification of dorsal cell fates in the vertebrate central nervous system. Annu. Rev. Neurosci. 22, 261–294 (1999).
Solloway, M. J. & Robertson, E. J. Early embryonic lethality in Bmp5;Bmp7 double mutant mice suggests functional redundancy within the 60A subgroup. Development 126, 1753–1768 (1999).
Grove, E. A., Tole, S., Limon, J., Yip, L. & Ragsdale, C. W. The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development 125, 2315–2325 (1998).
Lee, S. M., Tole, S., Grove, E. & McMahon, A. P. A local Wnt-3a signal is required for development of the mammalian hippocampus. Development 127, 457–467 (2000).
Galceran, J., Miyashita-Lin, E. M., Devaney, E., Rubenstein, J. L. & Grosschedl, R. Hippocampus development and generation of dentate gyrus granule cells is regulated by LEF1. Development 127, 469–482 (2000).
Ikeya, M., Lee, S. M., Johnson, J. E., McMahon, A. P. & Takada, S. Wnt signalling required for expansion of neural crest and CNS progenitors. Nature 389, 966–970 (1997).
Bishop, K. M., Goudreau, G. & O'Leary, D. D. Regulation of area identity in the mammalian neocortex by Emx2 and Pax6. Science 288, 344–349 (2000).
Mallamaci, A., Muzio, L., Chan, C. H., Parnavelas, J. & Boncinelli, E. Area identity shifts in the early cerebral cortex of Emx2−/− mutant mice. Nat. Neurosci. 3, 679–686 (2000).
Brunelli, S. et al. Germline mutations in the homeobox gene EMX2 in patients with severe schizencephaly. Nat. Genet. 12, 94–96 (1996).
Capra, V. et al. Schizencephaly: surgical features and new molecular genetic results. Eur. J. Pediatr. Surg. 6 Suppl 1, 27–29 (1996).
Granata, T. et al. Familial schizencephaly associated with EMX2 mutation. Neurology 48, 1403–1406 (1997).
Warren, N. et al. The transcription factor, Pax6, is required for cell proliferation and differentiation in the developing cerebral cortex. Cereb. Cortex 9, 627–635 (1999).
Sisodiya, S. M. et al. PAX6 haploinsufficiency causes cerebral malformation and olfactory dysfunction in humans. Nat. Genet. 28, 214–216 (2001).
Glaser, T. et al. PAX6 gene dosage effect in a family with congenital cataracts, aniridia, anophthalmia and central nervous system defects. Nat. Genet. 7, 463–471 (1994).
Gulisano, M., Broccoli, V., Pardini, C. & Boncinelli, E. Emx1 and Emx2 show different patterns of expression during proliferation and differentiation of the developing cerebral cortex in the mouse. Eur. J. Neurosci. 8, 1037–1050 (1996).
Xu, Y. et al. LH-2: a LIM/homeodomain gene expressed in developing lymphocytes and neural cells. Proc. Natl. Acad. Sci. USA 90, 227–231 (1993).
Donoghue, M. J. & Rakic, P. Molecular gradients and compartments in the embryonic primate cerebral cortex. Cereb. Cortex 9, 586–600 (1999).
Goodman, C. S. Mechanisms and molecules that control growth cone guidance. Annu. Rev. Neurosci. 19, 341–377 (1996).
Suzuki, S. C., Inoue, T., Kimura, Y., Tanaka, T. & Takeichi, M. Neuronal circuits are subdivided by differential expression of type-II classic cadherins in postnatal mouse brains. Mol. Cell. Neurosci. 9, 433–447 (1997).
Rubenstein, J. L. et al. Genetic control of cortical regionalization and connectivity. Cereb. Cortex 9, 524–532 (1999).
Vanderhaeghen, P. et al. A mapping label required for normal scale of body representation in the cortex. Nat. Neurosci. 3, 358–365 (2000).
Pimenta, A. F. et al. The limbic system-associated membrane protein is an Ig superfamily member that mediates selective neuronal growth and axon targeting. Neuron 15, 287–297 (1995).
Mann, F., Zhukareva, V., Pimenta, A., Levitt, P. & Bolz, J. Membrane-associated molecules guide limbic and nonlimbic thalamocortical projections. J. Neurosci. 18, 9409–9419 (1998).
O'Leary, D. D. & Wilkinson, D. G. Eph receptors and ephrins in neural development. Curr. Opin. Neurobiol. 9, 65–73 (1999).
Donoghue, M. J. & Rakic, P. Molecular evidence for the early specification of presumptive functional domains in the embryonic primate cerebral cortex. J. Neurosci. 19, 5967–5979 (1999).
Feng, G. et al. Roles for ephrins in positionally selective synaptogenesis between motor neurons and muscle fibers. Neuron 25, 295–306 (2000).
Prakash, N. et al. Malformation of the functional organization of somatosensory cortex in adult ephrin-A5 knock-out mice revealed by in vivo functional imaging. J. Neurosci. 20, 5841–5847 (2000).
Toresson, H., Potter, S. S. & Campbell, K. Genetic control of dorsal–ventral identity in the telencephalon: opposing roles for Pax6 and Gsh2. Development 127, 4361–4371 (2000).
Corbin, J. G., Gaiano, N., Machold, R. P., Langston, A. & Fishell, G. The Gsh2 homeodomain gene controls multiple aspects of telencephalic development. Development 127, 5007–5020 (2000).
Yun, K., Potter, S. & Rubenstein, J. L. Gsh2 and Pax6 play complementary roles in dorsoventral patterning of the mammalian telencephalon. Development 128, 193–205 (2001).
Guerreiro, M. M. et al. Familial perisylvian polymicrogyria: a new familial syndrome of cortical maldevelopment. Ann. Neurol. 48, 39–48 (2000).
Barkovich, A. J., Hevner, R. & Guerrini, R. Syndromes of bilateral symmetrical polymicrogyria. Am. J. Neuroradiol. 20, 1814–1821 (1999).
Straussberg, R., Gross, S., Amir, J. & Gadoth, N. A new autosomal recessive syndrome of pachygyria. Clin. Genet. 50, 498–501 (1996).
Zhao, Q., Behringer, R. R. & de Crombrugghe, B. Prenatal folic acid treatment suppresses acrania and meroanencephaly in mice mutant for the Cart1 homeobox gene. Nat. Genet. 13, 275–283 (1996).
Altman, J. & Bayer, S. A. Atlas of Prenatal Rat Brain Development 589 (CRC Press, Boca Raton, Florida, 1995).
Barkovich, A. J., Kuzniecky, R. I., Bollen, A. W. & Grant, P. E. Focal transmantle dysplasia: a specific malformation of cortical development. Neurology 49, 1148–1152 (1997).
Lai, C. S., Fisher, S. E., Hurst, J. A., Vargha-Khadem, F. & Monaco, A. P. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature 413, 519–523 (2001).
Acknowledgements
We thank L. Flanagan, M. Nieto, and E. Olson for comments on the manuscript. E.S.M. is supported by the NIMH, and C.A.W. is supported by the NINDS and the March of Dimes.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Monuki, E., Walsh, C. Mechanisms of cerebral cortical patterning in mice and humans. Nat Neurosci 4 (Suppl 11), 1199–1206 (2001). https://doi.org/10.1038/nn752
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn752
This article is cited by
-
Cell-cycle control and cortical development
Nature Reviews Neuroscience (2007)