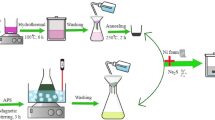
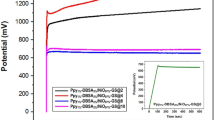
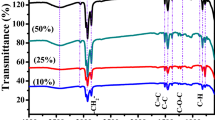
Abstract
Electrochemical supercapacitors can deliver high levels of electrical power and offer long operating lifetimes1,2,3,4,5,6,7,8, but their energy storage density is too low for many important applications2,3. Pseudocapacitive transition-metal oxides such as MnO2 could be used to make electrodes in such supercapacitors, because they are predicted to have a high capacitance for storing electrical charge while also being inexpensive and not harmful to the environment9,10. However, the poor conductivity of MnO2 (10–5–10–6 S cm–1) limits the charge/discharge rate for high-power applications10,11. Here, we show that hybrid structures made of nanoporous gold and nanocrystalline MnO2 have enhanced conductivity, resulting in a specific capacitance of the constituent MnO2 (∼1,145 F g–1) that is close to the theoretical value9. The nanoporous gold allows electron transport through the MnO2, and facilitates fast ion diffusion between the MnO2 and the electrolytes while also acting as a double-layer capacitor. The high specific capacitances and charge/discharge rates offered by such hybrid structures make them promising candidates as electrodes in supercapacitors, combining high-energy storage densities with high levels of power delivery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Winter, M. & Brodd, R. J. What are batteries, fuel cells, and supercapacitors? Chem. Rev. 104, 4245–4269 (2004).
Conway, B. E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications (Kluwer, 1999).
Simon, P. & Gogotsi, Y. Materials for electrochemical capacitors. Nature Mater. 7, 845–854 (2008).
Aricò, A. S., Bruce, P., Scrosati, B., Tarascon, J. M. & Van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nature Mater. 4, 366–377 (2005).
Miller, J. R. & Simon P. Electrochemical capacitors for energy management. Science 321, 651–652 (2008).
Pech, D. et al. Ultrahigh-power micrometer-sized supercapacitors based on onion-like carbon. Nature Nanotech. 5, 651–654 (2010).
Chmiola, J., Largeot, C., Taberna, P. L., Simon, P. & Gogotsi, Y. Monolithic Carbide-derived carbon films for micro-supercapacitors. Science 328, 480–483 (2010).
Huang, J. S., Sumpter, B. G. & Meunier, V. Theoretical model for nanoporous carbon supercapacitors. Angew. Chem. Int. Ed. 47, 520–524 (2008).
Toupin, M., Brousse, T. & Bélanger, D. Charge storage mechanism of MnO2 electrode used in aqueous electrochemical capacitor. Chem. Mater. 16, 3184–3190 (2004).
Chang, J. K. & Tsai, W. T. Material characterization and electrochemical performance of hydrous manganese oxide electrodes for use in electrochemical pseudocapacitors. J. Electrochem. Soc. 150, A1333–A1338 (2003).
Bélanger, D., Brousse, T. & Long, J. W. Manganese oxides: battery materials make the leap to electrochemical capacitors. Electrochem. Soc. Interface 17, 49–52 (2008).
Conway, B. E., Birss, V. & Wojtowicz, J. The role and utilization of pseudocapacitance for energy storage by supercapacitors. J. Power Sources 66, 1–14 (1997).
Rudge, A., Davey, J., Raistrick, I., Gottesfeld, S. & Ferraris, J. P. Conducting polymers as active materials in electrochemical capacitors. J. Power Sources 47, 89–107 (1994).
Chmiola, J. et al. Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer. Science 313, 1760–1763 (2006).
Kaempgen, M., Chan, C. K., Ma, J., Cui, Y. & Gruner, G. Printable thin film supercapacitors using single-walled carbon nanotubes. Nano Lett. 9, 1872–1876 (2009).
Pushparaj, V. L. et al. Flexible energy storage devices based on nanocomposite paper. Proc. Natl Acad. Sci. USA 104, 13574–13577 (2007).
Wu, M. Q., Snook, G. A., Chen, G. Z. & Fray, D. J. Redox deposition of manganese oxide on graphite for supercapacitors. Electrochem. Commun. 6, 499–504 (2004).
Yan, J. et al. Carbon nanotube/MnO2 composites synthesized by microwave-assisted method for supercapacitors with high power and energy densities. J. Power Sources 194, 1202–1207 (2009).
Reddy, A. L. M., Shaijumon, M. M., Gowda, S. R. & Ajayan, P. M. Multisegmented Au–MnO2/carbon nanotube hybrid coaxial arrays for high-power supercapacitor applications. J. Phys. Chem. C 114, 658–663 (2010).
Hu, L. B. et al. Stretchable, porous, and conductive energy textiles. Nano Lett. 10, 708–714 (2010).
Lei, Y., Fournier, C., Pascal, J. L. & Favier, F. Mesoporous carbon–manganese oxide composite as negative electrode material for supercapacitors. Micropor. Mesopor. Mater. 110, 167–176 (2008).
Zhou, R. F. et al. High-performance supercapacitors using a nanoporous current collector made from super-aligned carbon nanotubes. Nanotechnology 21, 345701 (2010).
Liu, R. & Lee, S. B. MnO2/poly(3,4-ethylenedioxythiophene) coaxial nanowires by one-step coelectrodeposition for electrochemical energy storage. J. Am. Chem. Soc. 130, 2942–2943 (2008).
Chen, L. et al. Synthesis and pseudocapactive studies of composite films of polyaniline and manganese oxide nanoparticles. J. Power Sources 195, 3742–3747 (2010).
Nakayama, M., Kanaya, T. & Inoue, R. Anodic deposition of layered manganese oxide into a colloidal crystal template for electrochemical supercapacitor. Electrochem. Commun. 9, 1154–1158 (2007).
Bruce, P. G., Scrosati, B. & Tarascon, J. M. Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. 47, 2930–2946 (2008).
Fujita, T. et al. Unusually small electrical resistance of three-dimensional nanoporous gold in external magnetic fields. Phys. Rev. Lett. 101, 166601 (2008).
Erlebacher, J., Aziz, M. J., Karma, A., Dimitrov, N. & Sieradzki, K. Evolution of nanoporosity in dealloying. Nature 410, 450–453 (2001).
Potapov, P. L., Jorissen, K. & Schryvers D. Effect of charge transfer on EELS integrated cross sections in Mn and Ti oxides. Phys. Rev. B 70, 045106 (2004).
Zhou, Y. K., He, B. L., Zhang, F. B. & Li, H. L. Hydrous manganese oxide/carbon nanotube composite electrodes for electrochemical capacitors. J. Solid State Electrochem. 8, 482–487 (2004).
Hou, Y., Cheng, Y. W., Hobson, T. & Liu J. Design and synthesis of hierarchical MnO2 nanospheres/carbon nanotubes/conducting polymer ternary composite for high performance electrochemical electrodes. Nano Lett. 10, 2727–2733 (2010).
Lee, S. W., Kim, J., Chen, S., Hammond, P. T. & Shao-Horn, Y. Carbon nanotube/manganese oxide ultrathin film electrodes for electrochemical capacitors. ACS Nano 4, 3889–3896 (2010).
Fischer, A. E., Saunders, M. P., Pettigrew, K. A., Rolison, D. R. & Long, J. W. Electroless deposition of nanoscale MnO2 on ultraporous carbon nanoarchitectures: correlation of evolving pore–solid structure and electrochemical performance. J. Electrochem. Soc. 155, A246–A252 (2008).
Lee, S. W., Kim, B. S., Chen, S., Shao-Horn, Y. & Hammond, P. T. Layer-by-layer assembly of all carbon nanotube ultrathin films for electrochemical applications. J. Am. Chem. Soc. 131, 671–679 (2009).
Pang, S. C., Anderson, M. A. & Chapman, T. W. Novel electrode materials for thin-film ultracapacitors: comparison of electrochemical properties of sol–gel-derived and electrodeposited manganese dioxide. J. Electrochem. Soc. 147, 444–450 (2000).
Acknowledgements
This work was sponsored by the Global COE for Materials Research and Education, the World Premier International (WPI) Research Center Initiative for Atoms, Molecules and Materials, and the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT). X.Y.L was supported by the Japan Society for the Promotion of Science (JSPS) postdoctoral fellowship programme (P07373).
Author information
Authors and Affiliations
Contributions
X.Y.L. and M.W.C. conceived and designed the experiments. X.Y.L. carried out the fabrication of materials and performed electrochemical characterization. A.H. and T.F. contributed to microstructural characterization. X.Y.L. and M.W.C. wrote the paper, and all authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1193 kb)
Rights and permissions
About this article
Cite this article
Lang, X., Hirata, A., Fujita, T. et al. Nanoporous metal/oxide hybrid electrodes for electrochemical supercapacitors. Nature Nanotech 6, 232–236 (2011). https://doi.org/10.1038/nnano.2011.13
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2011.13
This article is cited by
-
Construction of chitosan-supported nickel cobaltite composite for efficient electrochemical capacitor and water-splitting applications
Scientific Reports (2024)
-
Preparation of high-performance manganese-based pseudocapacitor material by using spent lithium-ion battery anode graphite via mechanochemical pretreatment
Carbon Neutrality (2024)
-
Composite Nanoarchitectonics Towards Method for Everything in Materials Science
Journal of Inorganic and Organometallic Polymers and Materials (2024)
-
Ti3C2Tx MXene as a growth template for amorphous RuOx in carbon nanofiber-based flexible electrodes for enhanced pseudocapacitive energy storage
NPG Asia Materials (2023)
-
Economical synthesized Mn3O4/biomass-derived carbon from vegetable sponge composites and its excellent supercapacitive behavior
Biomass Conversion and Biorefinery (2023)