Abstract
Chromatin remodeling, including histone modification, is involved in stimulant-induced gene expression and addiction behavior. To further explore the role of dopamine D1 receptor signaling, we measured cocaine-related locomotor activity and place preference in mice pretreated for up to 10 days with the D1 agonist SKF82958 and/or the histone deacetylase inhibitor (HDACi), sodium butyrate. Cotreatment with D1 agonist and HDACi significantly enhanced cocaine-induced locomotor activity and place preference, in comparison to single-drug regimens. However, butyrate-mediated reward effects were transient and only apparent within 2 days after the last HDACi treatment. These behavioral changes were associated with histone modification changes in striatum and ventral midbrain: (1) a generalized increase in H3 phosphoacetylation in striatal neurons was dependent on activation of D1 receptors; (2) H3 deacetylation at promoter sequences of tyrosine hydroxylase (Th) and brain-derived neurotrophic factor (Bdnf) in ventral midbrain, together with upregulation of the corresponding gene transcripts after cotreatment with D1 agonist and HDACi. Collectively, these findings imply that D1 receptor-regulated histone (phospho)acetylation and gene expression in reward circuitry is differentially regulated in a region-specific manner. Given that the combination of D1 agonist and HDACi enhances cocaine-related sensitization and reward, the therapeutic benefits of D1 receptor antagonists and histone acetyl-transferase inhibitors (HATi) warrant further investigation in experimental models of stimulant abuse.
Similar content being viewed by others
INTRODUCTION
To date, the molecular mechanisms that underlie cocaine and other stimulant addiction have become increasingly clear but current pharmacological treatment options remain surprisingly limited. Therefore, it will be important to further elucidate the molecular pharmacology of cocaine addiction in preclinical models. Among the various drug-based interventions that modulate the animal's response to cocaine are two radically different classes of compounds—dopamine D1 receptor agonists and histone protein deacetylase inhibitors (HDACi). Notably, D1 agonist drugs elicit reward functions, including reinstatement of cocaine-related addiction behavior (Self and Stein, 1992; Graham et al, 2007) and the importance of D1-mediated signaling in the neurobiology of stimulant abuse is established by various genetic and pharmacological approaches (Hummel and Unterwald, 2002). Importantly, the neurochemical and behavioral adaptations in response to stimulant or D1 agonist exposure are thought to result, at least in part, from changes in gene expression affecting dopaminergic circuitry, including ventral midbrain and the striatum and other major target zones within the forebrain. Recently, it was recognized that among these cocaine-sensitive transcriptional mechanisms, histone acetylation and other chromatin-remodeling events are of particular importance; for example, pharmacological inhibition or genetic ablation of class I/II histone deacetylases enhances cocaine-related behavioral sensitization and reward (Kumar et al, 2005; Renthal et al, 2007). Conversely, striatal overexpression of histone deacetylase (HDAC) or genetic ablation of histone acetyl transferases, including Cbp, attenuates the response of the animal to cocaine (Kumar et al, 2005; Levine et al, 2005).
However, it is not known whether or not these two reward-enhancing drug types—D1 receptor agonists and HDAC inhibitors—operate synergistically or independently. Therefore, it was the goal of this study to understand how drug-induced changes in D1 receptor signaling and histone deacetylase activity modulate the behavioral and molecular responses of the animal to cocaine. We applied, in C57Bl6/J mice, two cocaine-related behavioral assays; locomotor sensitization and place preference. The molecular studies were conducted on ventral midbrain at the level of the substantia nigra/ventral tegmental area (SN/VTA) and striatum (including but not limited to the nucleus accumbens) (Winder et al, 2002; Kalivas and Volkow, 2005)—two key regions of the reward circuitry of the brain. Specifically, we examined drug-induced changes in nucleosome core histone H3 and H4 acetylation and phospho-acetylation, two types of modifications previously linked to changes in dopaminergic signaling (Li et al, 2004; Brami-Cherrier et al, 2005; Kumar et al, 2005; Levine et al, 2005; Santini et al, 2007).
Our results indicate that combined D1 receptor activation and HDAC inhibition was associated with a heterogeneous set of region-specific chromatin-remodeling mechanisms, including a generalized histone phosphoacetylation response in striatal neurons and histone deacetylation in SN/VTA chromatin at promoters of tyrosine hydroxylase (Th) and brain-derived neurotrophic factor (Bdnf)—two genes with a key role in drug-induced plasticity (Self et al, 2004; Corominas et al, 2007). However, changes in cocaine-related behaviors after previous exposure to D1 agonist with or without HDACi cotreatment were complex, because the two different types of drugs exerted a synergistic effect in some but not all behavioral assays. These differences were, at least in part, explained by the observation that butyrate-mediated reward effects were transient and only apparent within 2 days after the last HDACi treatment.
MATERIALS AND METHODS
Drugs
SB or N-butyric acid sodium salt, and SKF or chloro-APB hydrobromide (±)-SKF-82958 hydrobromide or cocaine (Sigma-Aldrich, St Louis, MO) were dissolved in saline and prepared fresh each day. For this study, SKF was chosen as a full D1-like agonist and SB was chosen as a class I/II HDAC inhibitor. Other drugs included in this study were the PKA inhibitor N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide dihydrochloride (H89) and the NMDA receptor antagonist [(+)MK 801 hydrogen maleate] (MK-801), both dissolved in DMSO.
Animals
All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Massachusetts. For all experiments with the D1 receptor agonist SKF82958, male C57BL/6J mice, aged 9–15 weeks, were used. Animals were purchased directly from the Jackson Laboratory, Bar Harbor (Maine), and housed in groups of 2–4 per cage with food and water ad libitum under 12-hour light/dark cycle. For behavioral experiments, mice received i.p. injections with SB (100 or 25 mg/kg) or saline followed 15 min later by SKF (0.25 mg/kg) or saline once daily for 10 days. For the remaining experiments, mice received i.p. injections with SKF (1 mg/kg) or saline as vehicle, with or without concomitant SB (200 or 25 mg/kg), as single dose or once daily for 2–10 days.
For tissue extraction, animals were killed by cervical dislocation/decapitation and whole brain was removed and processed immediately or snap-frozen on dry ice. Striatum was dissected unilaterally from coronal slices positioned between Bregma 1.18 and −0.46 mm; ventral midbrain was dissected from coronal slices positioned between Bregma −2.80 and −3.80 mm and ventral to the dorsal third ventricle; the dissected blocks of tissue thus included the full rostro-caudal and dorso-ventral extent of the SN/VTA (Paxinos and Franklin, 2001).
Behavioral Testing
Tests were conducted during the light cycle between 0730 and 1430 h. Depending on the drug and test, 8–10 animals per experiment were used, with a matched number of saline-treated control subjects.
Locomotor activity
Animals were placed in clean, 19.1 × 29.2 × 12.7 cm shoebox-style cages (Allentown Inc., Allentown, NJ) and locomotor activity measured continuously for 190 min (from t=0 to t=190 min) using a photobeam activity system (San Diego Instruments, San Diego, CA). All mice were placed in activity cages and allowed to habituate for 75 min prior to any injection. To assay the effects of SB and SKF pretreatment on cocaine-induced locomotion, mice were first introduced to the test and injected with saline at t=90 min to record basal activity (day 0). The next day, a 10-day pretreatment period was initiated with daily i.p. injections of (i) Sal+SKF; (ii) SB+SKF; (iii) SB+Sal; or (iv) Sal+Sal, each at t=75 and t=90 min, respectively. During pretreatment, activity was measured on days 1, 2, 5, 7, and 10. On day 11, animals were placed in activity cages, saline injected at t=75 min followed by cocaine (15 mg/kg) at t=90 min, and activity measured for 190 min total.
Conditioned place preference
The conditioned place preference (CPP) test apparatus consisted of a rectangular cage with overall inside dimensions of 46.5 × 12.7 × 12.7 cm (med-associates, St Albans, VT). This included a center neutral gray compartment, 7.2 cm long, and a hinged clear polycarbonate lid for loading the test animal. The adjacent conditioning compartments measured 16.8 cm long. One compartment was black with a stainless steel grid rod floor and the other white with a square stainless steel mesh floor. Chambers were equipped with photobeams to measure activity and time spent in each chamber. Guillotine doors separated the chambers and could be fixed in closed or opened position. The apparatus was placed in a sound attenuation cubicle.
Two cocaine-related CPP paradigms were used in the present study, with the main difference being the time line of SKF and SB treatments.
Paradigm 1: Preceding CPP testing, all animals were treated for 10 days with daily injections of Sal+SKF or SB+SKF or Sal+Sal as described above. Then, CPP was tested in three stages over a 6-day period. In the first stage, each mouse was placed in the central compartment and allowed free access to all chambers. The time spent in each chamber was then recorded over a 20-min period. During the second stage, i.p. injections of cocaine (5 mg/kg) were paired with one of the conditioning chambers and saline injections paired with the other. Each mouse received cocaine injections and was placed in the isolated cocaine-conditioning chamber on days 3 and 5; each mouse received saline injections and was placed in the saline chamber on days 2 and 4. Each training trial lasted 20 min. During stage 3, the testing phase, each mouse was once again given free access to all chambers for 20 min. The time spent in each chamber during stage 1 (baseline) was subtracted from the time spent in each chamber during stage 3. A preference toward the cocaine-associated chamber compared to baseline is a measure of the reward behavior associated with cocaine. Control mice received saline in both chambers. Mice that exhibited an initial bias for the white or black chamber received cocaine in the least-preferred chamber.
Paradigm 2: In contrast to paradigm 1, no pretreatment was administered and animals were subjected to the first (baseline, day 1) and second stage (saline, days 2 and 4; cocaine, days 3 and 5) of CPP training as described above. In addition, on days 1, 2, and 4, animals received within 1 h after the training session, SKF±SB at the same doses described above.
Cell Culture Studies
Primary striatal cultures were prepared as described previously, with minor modifications (Konradi et al, 1996; Rajadhyaksha et al, 1998). Striata from 18-day-old Sprague–Dawley rat fetuses were dissected and resuspended in defined medium (50% F12/DMEM and 50% DMEM (Gibco-Invitrogen, Grand Island, NY) with the following supplements per liter of medium: 4 g of dextrose, 1 × B27, 10 ml of penicillin–streptomycin liquid (Gibco-Invitrogen), and 25 mM HEPES). Cells were resuspended in defined medium to 1.2 × 106 cells/ml and plated in 12-well plates (Costar, Cambridge, MA) at 2 × 106 cells per well. Plates were pretreated with 1 ml of a 1 : 500-diluted sterile solution of polyethylenimine in water for 18 h, washed twice with sterile water, coated with 2.5% serum-containing PBS solution for at least 4 h, and aspirated just before plating. All experiments were performed in triplicate with cells 6 days in culture and repeated at least once in an independent dissection. As determined by HPLC analysis, glutamate levels in the medium on the day of the experiments ranged from 1 to 5 μM. The neuron-to-astroglia ratio was below 25 : 1, as established by immunocytochemical staining with the glial fibrillary acid protein (Dako, Carpinteria, CA) and counterstaining with 1% cresyl violet. All drugs used for the in vitro experiments were purchased from Sigma. At two different time points prior to harvest—3 h and 30 min—cells were treated with one of the following drugs (treatment no. 1): (1.1) vehicle (10 μl DMSO); (1.2) N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide dihydrochloride (H89), 20 μM; (1.3) the NMDA receptor antagonist [(+)MK 801 hydrogen maleate] (MK-801), 2 μM. Three hours before harvest, cells were treated with one of the following drugs (treatment no. 2): (2.1) vehicle (10 μl water); (2.2) sodium butyrate (10 μM to 10 mM); (2.3) SKF82958 (50 μM).
Western Blotting
Striatal tissue was homogenized in Laemmli buffer, then incubated at 37°C for 10 min, centrifuged at 13 500g at 4°C; the supernatant was denatured at 95°C for 5 min, then electrophoresed on a 10–20% linear gradient Tris-HCl gel (Bio-Rad) and transferred to PVDF membrane (0.2 μM pore size, Bio-Rad). Immunolabelings were performed with antiphospho (Ser10)-acetyl (Lys14)-histone H3 antibody (no. 07–081), antiphospho (Ser10)-histone H3 antibody (no. 05–598), anti-acetyl histone H3 antibody (no. 06–599), and as loading control, a modification-independent antihistone H4 antibody (no. 07–108), all from Upstate, Lake Placid, NY. Additional blots were probed with anti-Th (no. T-2928), Sigma-Aldrich and antiphospho (Ser31)-Th (no. 36–9900), Zymed Laboratories-Invitrogen. Immunoreactivity was detected using peroxidase-conjugated secondary antibody (donkey anti-rabbit IgG or sheep anti-mouse; Amersham) in conjunction with chemiluminescence-based film autoradiography (Super Signal West Dura Extend Reagent; Pierce). For quantification, Quantity One software was used (Bio-Rad).
Immunohistochemistry
Coronal sections (14 μm) were cut from blocks containing striatum and collected in ice-cold PBS for free-floating immunohistochemistry. Sections were blocked in 0.3% Triton X-100/±2% goat serum/0.1 M sodium phosphate (pH 7.4). Sections were next incubated in 0.3% Triton X-100/0.1 M sodium phosphate buffer and the antiphospho (ser10)-acetyl (Lys9)-histone H3 antibody (1 : 500, no. ab4272; Abcam, Cambridge, MA) together with the monoclonal anti-NeuN antibody (1 : 300, no. MAB377; Chemicon, Temecula, CA) overnight at 4°C, washed, then incubated with Texas Red-conjugated horse anti-mouse antiserum and FITC-conjugated goat anti-rabbit antiserum (1 : 200; Vector) for 60 min at RT, washed, slide-mounted, dried, counterstained with 4,6,-diamidino-2-phenylindole dihydrochloride (DAPI), and coverslipped with Vectashield (Vector). Sections were examined with a Zeiss Axiovert microscope and digitized images were obtained with OpenLab software (Improvision, Lexington, MA).
Quantitative RT-PCR
From each animal, total RNA from striatum or ventral midbrain was extracted using the trizol-based RNeasy Lipid Tissue mini kit (Qiagen). Amplification reactions were performed in triplicate, using the ABI PRISM 7500 Real Time PCR System (Applied Biosystems) in conjunction with the One-Step RT-PCR kit (Applied Biosystems), SYBR green as a reference dye, and the following cycling protocol: 48°C, 30 min; 95°C, 10 min; followed by 45 cycles of 95°C 15 s, 60°C 1 min. Primer sequences were designed to amplify 90–150 bp fragments from respective gene-coding regions: Th primer sequences (94 bp product): forward, attggaggctgtggtattcg ; reverse, cgagacagtgaggagggttt ; Bdnf primer sequences (118 bp product): forward, gcgcccatgaaagaagtaaa ; reverse, tcgtcagacctctcgaacct ; C-fos primer sequences (147 bp product): forward, atccttggagccagtcaaga ; reverse, gcatagaaggaaccggacag ; β2-microglobulin (B2m) primer sequences (98 bp product): forward, tggtgcttgtctcactgacc ; reverse, tatgttcggcttcccattct . A 133 bp fragment from mouse 18S rRNA was amplified in parallel reactions for normalization. 18S rRNA primer sequences used were forward, catggccgttcttagttggt ; reverse, gaacgccacttgtccctcta . For additional information on PCR primers and products, see Supplementary Table. Quantification and normalization procedures were previously described (Stadler et al, 2005).
Chromatin Immunoprecipitation and Quantitative PCR
Ventral midbrain tissue (30 mg, pooled from 2–3 mice) was homogenized and digested with micrococcal nuclease for further application to native chromatin immunoprecipitation (ChIP) with anti-acetyl histone H3 (Upstate no. 06–599) or anti-acetyl histone H4 (Upstate no. 06–598) antibodies or modification-independent anti-H4 (Upstate no. 07–108) and anti-H3 (Abcam Ab1791–100) followed by quantitative PCR (qPCR) as previously described (Huang et al, 2006). Primers were selected upstream of respective gene trascription start sites (TSS), with product sizes in the range of 75–125 bp to amplify from mononucleosomal DNA (147 bp). Three primers were designed to amplify sequences proximal to the Th promoter region: Th-a forward, ttgaagacacagcctgcaac ; reverse, ggggaggtcagaagacccta (70 bp product, −652 bp from TSS=150085865; NC_000073.5); Th-b forward, acggaggcctctctcgtc ; reverse, gtcccccacctcctacctc (103 bp product, −91 bp from TSS=150085865; NC_000073.5); and Th-c forward, ctggggtatccacccattta ; reverse, gccgtctcagagcaggatac (113 bp product, +48 bp from TSS=150085865; NC_000073.5). Three primers were designed to amplify sequences from the non-coding BDNF promoter regions pI, pIII, and pIV: pI (106 bp product) forward, tggagacccttagtcatggtg ; reverse, gcctctctgagccagttacg ; pIII (129 bp product) forward, aatgtgcagtggggaaagag ; reverse, ggcagggataccgagagaat ; pIV (114 bp product) forward, aaatggagcttctcgctgaa ; reverse, agtctttggtggccgatatg . Primers were designed for the housekeeping gene B2m, forward, gggaaagtccctttgtaacct ; reverse, gcgcgcgctcttatatagtt (112 bp product, −57 bp from TSS=121973475; NC_000068.6). For additional information on PCR primers and products, see Supplementary Table.
Statistical Analysis
Biochemical data (immunoblots, qPCR) from experiments with two groups were compared using Student's t-test. For experiments with three or more groups, comparisons were made with one-way ANOVA and the Tukey–Kramer or Least-Significant Differences tests for post-hoc comparison (SYSTAT). Significance in all behavior experiments was determined by one- or two-way ANOVA followed by post-hoc analysis (Tukey test unless otherwise stated).
RESULTS
Systemic Pretreatment with D1 Agonist and HDACi Enhances Cocaine-Induced Locomotor Activity and Reward
We first examined whether cocaine-induced locomotion is affected by previous exposure to the D1/D5 agonist, SKF82958. The drug was administered once daily (0.25 mg/kg i.p.) for 10 days and 24 h after the last dose, animals received a single dose of cocaine (15 mg/kg i.p.). Comparison of locomotor activity of mice pretreated for 1–10 days with SKF±SB (two-way ANOVA) revealed significant main effects due to treatment (FSB+SKF vs Sal+SKF (1, 70)=10.1, p<0.01) and time (FSB+SKF vs Sal+SKF (4, 70)=13.3, p<0.001), but no significant interactive effect between treatment and time (FSB+SKF vs Sal+SKF (4, 70)=0.962, p=0.434) (Figure 1a). Furthermore, in comparison to animals pretreated with saline, exposure to D1 agonist resulted in a more than twofold increase in cocaine-induced locomotion (Figure 1b), which is in agreement with previous studies (Pierce et al, 1996; De Vries et al, 1998; Sorg et al, 2004). Importantly, this cocaine-related locomotor sensitization was even further enhanced—up to fivefold when compared to saline controls—in animals pretreated for 10 days with D1 agonist plus SB (100 mg/kg) (Figure 1b). The cocaine-induced activity in the group cotreated with 100 mg/kg SB+SKF was also significantly increased compared to mice pretreated with SKF as single drug (Figure 1c) (FSB+SKF vs Sal+SKF (3, 28)=33.39, p<0.001). Notably, 100 mg/kg SB administered as single drug was indistinguishable from saline (Figure 1b and c). Furthermore, SB+SKF cotreatment with a lower dose of SB (25 mg/kg) was indistinguishable from the SKF single-drug regimen (Figure 1c). We conclude that pretreatment with D1 agonist and the HDACi, SB, results in a synergistic and dose-dependent locomotor activity effect of a subsequent dose of cocaine administered 24 h later.
Changes in cocaine-induced behavior after pretreatment with D1 agonist and HDACi. (a) Locomotor activity during pretreatment; Sal followed by SKF (filled square); SB followed by SKF (open square); SB followed by Sal (open circle) and Sal followed by Sal (filled circle). Data show locomotor activity for the first 60 min following second drug (mean±SEM, N=8–10 animals per group). Notice that locomotor activity was significantly increased in mice treated with SKF±SB when comparing activity on day 1 with days 2, 5, 7, or 10 (**p<0.001). (b) Locomotor activity before and after a single dose of cocaine. Animals were placed in activity cages 24 h after the last pretreatment (see (a)) and behavior was recorded for t=190 min total (x axis). Saline injected at t=75 min and cocaine (15 mg/kg) at t=90 min as indicated by arrows. Notice that animals pretreated with HDACi plus D1 agonist (SB+SKF; open squares) show a significant increase in cocaine-induced ambulation when compared to group pretreated with D1 agonist only (Sal+SKF; filled circles). (c) Total ambulation after cocaine (see also (b)). Notice that pretreatment with 100 mg/kg SB+SKF, but not 25 mg/kg SB+SKF, is associated with a significant, fourfold increase in cocaine-induced ambulation, and pretreatment with SKF (Sal+SKF) with a 2.5-fold increase, when compared to mice pretreated with saline only (Sal+Sal). (d and e) Conditioned place preference (d=paradigm 1, e=paradigm 2, see Materials and Methods), expressed as difference in time spent in cocaine- or saline-associated chamber before and after exposure (y axis). (d) Enhanced place preference in animals pretreated with Sal+SKF is similar to group treated with SB+SKF, when compared to mice pretreated with saline (Sal+Sal). (e) Significant cocaine-related place preference in group treated with SB+SKF, as compared to single drug SKF. *p<0.05; **p<0.01; n=8–10 per group; all data shown as mean±SEM.
Next, we wanted to examine whether or not previous exposure to D1 agonist and HDACi results in sustained (long-term) changes in cocaine-related reward behavior. To examine this, we treated three groups of mice for 10 days either with (i) saline, (ii) SKF 82958 (0.25 mg/kg), or (iii) SKF plus SB (100 mg/kg). Then, animals were subjected for a 6-day period to a place preference paradigm (CPP, paradigm 1, see Materials and Methods) to distinguish the rewarding effects of cocaine (5 mg/kg) from saline (see Materials and Methods for details). On the sixth and the final CPP day, animals that had been pretreated with SKF showed a significant increase in preference for the cocaine chamber (Figure 1d), which is in agreement with a recent report in rats (Graham et al, 2007). However, in this paradigm, cotreatment with SB was not more effective than single drug SKF (Figure 1d), and therefore we conclude that exposure to the D1 agonist but not the HDACi induced a sustained change in behavioral plasticity as it pertains to cocaine-related preference behavior.
The above CPP results suggest that repeated exposure to D1 agonist, but not SB, induces sustained changes in reward circuitry that lasted at least 6 days. Next, we wanted to find out whether or not SB enhances cocaine reward of animals that receive a much shorter course of SKF treatments. Therefore, in CPP paradigm 2, no pretreatment with SKF or SB was administered, and instead these drugs were administered during the training period and cocaine reward was evaluated 48 h after the last dose of SKF±SB (see Materials and Methods). Indeed, in paradigm 2, mice treated with SKF+SB showed significant cocaine-related place preference. This effect was not seen in animals that received single drug SKF (Figure 1e). We conclude that exposure to SB enhances cocaine-related reward of D1 agonist within a shorter time frame.
Exposure to D1 Agonist and HDACi is Associated with Increased Gene Expression in Ventral Midbrain and Chromatin Remodeling at Selected Promoter Sequences
To find out whether the increased cocaine-related locomotion in animals pretreated with SKF82958 and SB is associated with changes in gene transcription in ventral midbrain and striatum as key structures of the reward pathway, we assayed mRNA levels for (i) TH (Belin et al, 2007), the rate-limiting enzyme in catecholamine biosynthesis, (ii) BDNF, a nerve growth factor molecule thought to play an important role in the neurobiology of cocaine addiction (Guillin et al, 2001; Hall et al, 2003; Graham et al, 2007, iii) c-fos, a prototype early response gene, and (iv) β2-microglobulin, a ‘housekeeping gene’, as a control. For each gene (i–iv), transcript levels were determined by qRT-PCR and normalized to 18S rRNA levels, which differed less than 5–10% between groups, with no consistent effect by treatment.
Notably, animals that received 10 daily doses of SKF+100 mg/kg SB (ie, the treatment associated with the highest degree of locomotion upon subsequent exposure to cocaine, see Figure 1b and c) showed significantly higher levels of Th and Bdnf mRNA levels in ventral midbrain, in comparison to SKF+Sal-treated animals (F (3, 15)SB+SKF vs Sal+SKF=9.586TH, p<0.03;=8.891BDNF, p<0.04) (Figure 2A). In contrast, ventral midbrain B2m mRNA levels and all striatal transcripts were indistinguishable between animals that received SB or Sal as cotreatment together with SKF (Figure 2A). We conclude that the locomotor-activating effects of the HDACi, SB, are associated with transcriptional changes in ventral midbrain.
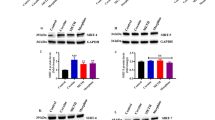
Treatment with D1 agonist and HDACi is associated with increased gene expression in ventral midbrain and chromatin remodeling at selected promoter sequences. (A) mRNA levels for Th, Bdnf, and B2m from striatum (Stri.) and ventral midbrain (Vent. Mid.) of animals treated for 10 days with Sal+SKF or SB+SKF or SB+Sal or Sal+Sal and killed 30 min after last injection. Data from qRT-PCR expressed as mean±SEM after normalization to 18S rRNA. Notice that ventral midbrain of animals treated with SB+SKF shows significant increases in Th, Bdnf transcripts compared to Sal+SKF and Sal+Sal. In contrast, ventral midbrain B2m levels were significantly decreased in SKF±SB mice. N=5 per group per brain region; *p<0.05; **p<0.01. (B) Top, schematic presentation of positioning of primer pairs used for ChIP studies at Th, Bdnf, and B2m promoters (left graph: Th promoter; (a–c) middle graph: Bdnf promoter I, III, IV; and right graph, B2m) for amplification of mono-nucleosomal DNA. TSS=transcription start site. Bar graphs showing levels of H3 acetylation at promoter sequences from ventral midbrain of mice after 10 daily treatment with SB+SKF (black bars) or Sal+Sal (white bars) as indicated. Animals were killed 30 min after last treatment. Data shown as chip-to-input ratios (mean±SEM, N=5–10 per group). Notice a significant decrease in histone acetylation at Th promoter sequence ‘a’ and Bdnf promoter IV following SB+SKF treatment (*p<0.05).
Next, we wanted to examine if the increases in ventral midbrain Th and Bdnf mRNA levels in the SKF+SB-treated animals are associated with chromatin-remodeling events at the corresponding promoters. Importantly, transcriptional profiling by microarray consistently revealed that after treatment with class I/II HDACi, a nearly equal number of genes show decreased or increased expression (Mariadason et al, 2000; Nusinzon and Horvath, 2005; Chen and Cepko, 2007). Furthermore, it is possible that—in addition to their established function in transcriptional repressor complexes (Butler and Bates, 2006)—HDAC activity is necessary to maintain transcriptional activity at selected genomic loci (Dehm et al, 2004). These novel findings suggest that both increased and decreased histone acetylation in a promoter chromatin environment could potentially be associated with increased gene expression levels. Therefore, we monitored histone H3 acetylation levels at Th, Bdnf, and B2m gene promoters. Immunoprecipitation of ventral midbrain prepared by micrococcal nuclease digestion showed that one out of three sites within close proximity to the Th transcription start site (Figure 2Ba–c) had a significant decrease in H3 acetylation in 1 mg/kg SKF+200 mg/kg SB-treated animals compared to saline controls. The H3 acetylation changes after cotreatment with SKF and the higher SB dose were specific because H4 acetylation at this portion of the Th promoter was unchanged (Supplementary Figure 1). This particular finding may not be too surprising given that differential regulation of H3 and H4 acetylation has been noted before both in cell lines (Rada-Iglesias et al, 2007) and in brain tissue (Kumar et al, 2005; Chwang et al, 2007). Moreover, SB-induced H3 acetylation changes were dose dependent, because cotreatments with 1 mg/kg SKF+25 mg/kg SB did not result in significant histone acetylation changes at the Th promoter (Supplementary Figure 2).
Furthermore, we measured histone H3 acetylation in chromatin surrounding Bdnf—a gene with complex transcriptional regulation, multiple promoters, and non-coding exons (Timmusk et al, 1993). For example, increased Bdnf expression after antidepressant treatment involves exon I (Russo-Neustadt et al, 2000; Dias et al, 2003). Moreover, dramatic increases in exon III and IV expression were observed in an animal model for social stress (Tsankova et al, 2006). Given this background, we determined H3 acetylation at the Bdnf for each promoter of the non-coding exons I, III, and IV. Chromatin from ventral midbrain of SB+SKF-treated mice showed, in comparison to saline-treated controls, no changes in H3 acetylation at Bdnf promoters PI and PIII (Figure 2B). However, ventral midbrain chromatin from the SKF+SB-treated animals showed a significant downregulation of H3 acetylation at Bdnf promoter IV at the higher SB dose of 200 mg/kg (Figure 2B) but not after 25 mg/kg SB (Supplementary Figure 2). Furthermore, changes in H4 acetylation were more variable (Supplementary Figure 1). Furthermore, there were no significant acetylation changes at the B2m promoter (Figure 2B). Taken together, our result shows that in ventral midbrain, upregulated expression of Th and Bdnf in response to D1 agonist and HDACi cotreatment is associated with histone H3 deacetylation events of selected portions of the corresponding gene promoters. Given that our immunoprecipitation assays were performed on mononucleosomal chromatin preparations from ventral midbrain (see Materials and Methods), we wanted to find out whether a decrease in nucleosomal densities underlies the observed histone hypoacetylation at selected sequences of the Th and Bdnf promoters in the SB+SKF-treated animals. We therefore conducted ventral midbrain ChIP with modification-independent anti-H4 or anti-H3 antibodies; however, SKF+SB-treated animals showed, in five out of six experiments, an increase in nucleosomal densities at the Th promoter sequence ‘a’ (see also Figure 2B) and similar changes at Bdnf promoter IV, in comparison to saline-treated controls (data not shown). Therefore, the observed decrease in histone acetylation—which was approximately 0.5-fold for Th and Bdnf (Figure 2B)—cannot be explained in terms of dispersion or decrease in nucleosome density.
Cotreatment with D1 Agonist and HDACi is Associated with a Transient Upregulation of Th Protein in Striatum
To find out whether the observed changes in gene expression and histone acetylation at the Th promoter in ventral midbrain occur in conjunction with alterations in TH protein levels, we assayed TH protein levels, including its phospho-activated form, phospho-Ser31-TH (Moy and Tsai, 2004). Animals were killed 30 min after acute or chronic treatment (see Materials and Methods), which corresponds to the time (post-injection) at which changes in locomotor activity were observed. We assayed two brain regions, the SN/VTA and the striatum, including nucleus accumbens and caudate-putamen, a cocaine-sensitive target for dopaminergic fibers (Graybiel et al, 1990; Winder et al, 2002). In the striatum, significant changes were limited to the group of animals treated with SB+SKF, which showed a twofold increase in TH immunoreactivity when compared to saline-treated controls (Figure 3a, (FSB+SKF vs Sal+Sal (3, 32)=2.936, p<0.01)). In contrast, animals treated with single drug SKF or SB did not show significant changes (Figure 3a). Likewise, significant changes in striatal phospho-Ser31-TH were limited to the group of SKF+SB-treated animals, which showed a 30% increase compared to animals treated with single drugs. These changes were significant (FSB+SKF vs SKF+Sal or SB+Sal (3, 32)=2.312, p<0.040) (data not shown). In contrast to the significant upregulation of striatal TH immunoreactivity in the SKF+SB-treated animals, levels of TH protein in ventral midbrain remained unaffected (Figure 3b). Furthermore, the observed increase in striatal TH after SKF+SB was transient because it was apparent at 30 min (Figure 3a) but not 6 days (Figure 3c) after the last of 10 daily treatments. We conclude that repeated exposure to D1 agonist and HDACi is associated with a transient increase in striatal TH protein. Furthermore, we assayed the concentration of dopamine and its metabolite, DOPAC, by HPLC and no significant differences between treatment groups were observed (Supplementary Figure 3). This would suggest that an increase in striatal TH (including phospho-TH) is not necessarily reflected by a net change in dopamine tissue levels.
A transient increase in striatal TH after exposure to D1 agonist and HDACi. (a) Top: representative immunoblots from striatum of animals treated for 10 days with Sal+SKF or SB+SKF or SB+Sal or Sal+Sal and killed 30 min after last injection. Blots were labeled with anti-TH (upper band) and antihistone H4 as loading control (lower band). Bottom: bar graph showing densitometric quantification of striatal immunoblots (mean±SEM, N=6–10 per group). Notice significant, twofold increase in TH protein levels in animals treated with SB+SKF compared to saline-treated controls. (b) TH immunoblots from ventral midbrain of animals treated with SB+SKF and Sal+Sal (same groups as shown in a) reveal no significant differences. (c) Striatal TH immunoblots from mice subjected to CPP (see also Figure 1d), animals were killed 6 days after last injection of SB+SKF and 24 h after last dose of cocaine. There is no significant difference between mice pretreated with SB+SKF as compared to pretreatment with Sal+Sal (N=3 per group) **p<0.01.
Activation of D1 Signaling is Linked to a Nucleosomal Response in Striatum
On the basis of the findings of the present study, repeated treatment with SKF+SB selectively induces gene expression changes and chromatin remodeling in ventral midbrain, and this occurs in conjunction with an increase in locomotor activity upon a subsequent dose of cocaine (Figure 1b–c). However, repeated exposure to the D1 agonist SKF—administered with or without the HDACi, SB—results in a sustained change in behavioral plasticity because it was linked to enhanced cocaine reward 6 days after the last dose of SKF (Figure 1d). As a first step towards understanding these phenomena, we wanted to identify a chromatin-remodeling mechanism linked to D1 signaling. Notably, previous studies reported D1 agonist or stress-induced changes in hippocampal histone phosphorylation and phosphoacetylation (Crosio et al, 2003; Chandramohan et al, 2007) and D2-like antagonist-induced changes in striatal histone phosphoacetylation (Li et al, 2004). Importantly, the kinetics of this generalized H3 phosphorylation and phosphoacetylation bears resemblance to the induction pattern of immediate-early genes (IEGs) and has been discussed as a ‘nucleosomal response’ in response to receptor activation and other stimuli (Thomson et al, 1999).
To examine the in vivo kinetics of striatal H3 phosphoacetylation (phospho-serine 10, acetyl lysine 14, H3pS10acK14)—a dual histone modification enriched in neuronal nuclei (Figure 4a)—in response to D1 receptor activation, we treated mice either with a single dose, or by repeated, daily injections (2 and 10 days) of SKF, with or without SB, and measured striatal H3pS10acK14 levels 30–120 min after the last dose. Levels of H3pS10acK14 showed a more than 2.5-fold increase after acute administration of SKF82958, and returned to baseline within 120 min after treatment (Figure 4b and c; (FSKF 30 min vs Sal (2, 13)=17.954, p<0.001)). These changes were specific for the dual modification, H3pS10acK14, because levels of the single modification, H3pS10, remained unchanged after SKF82958 treatment (Figure 4b). Similar changes were observed after repeated exposure for 10—but not 2—days of SKF when administered either as single drug or in combination with SB (Figure 4b and c). In contrast, single drug SB was indistinguishable from saline (Figure 4b and c). The transient resistance of the histone modification machinery to further induction by SKF82958 in the 2-day treatment paradigm (Figure 4b and c) was not due to tolerance, as striatal activation of the prototype early response gene, c-fos, was robust at all time points examined (Supplementary Figure 4).
D1 receptor signaling regulates H3 phosphoacetylation in striatal neurons. (a) Digitized images from striatal section processed for H3p-ac (red, upper panel), and NeuN (green, lower panel) immunofluorescence, with DAPI counterstain (blue). Notice robust H3p-ac signal in NeuN-positive cells (arrowheads) but absent in non-neuronal (NeuN-negative) cells (arrow). Images taken at 10 × 63 magnification. (b and c) Histone immunoblots and densitometric quantifications from striatum of mice treated with SB and SKF as single drug or cotreatments as indicated. Blots in (b) labeled with H3p-ac (upper panels) and H3p (lower panels), each with H4 as loading control. Notice robust increase in striatal H3 phosphoacetylation, both after acute and 10 days of treatment of SKF±SB. Animals were killed 30 min after last treatment. Bar graphs in (c) show densitometric quantifications (mean±SE) of striatal H3p-ac (N=3–5 per group). (d and e) Immunoblot (top) and densitometric quantification (bottom) from cultured striatal neurons showing changes in (d) H3p-ac and (e) H3ac after 3 h of treatment with SB and/or SKF. Notice (d) significant increase in H3p-ac after 3 h treatment with SB or SKF, with the highest levels in cultures cotreated with SB+SKF. In contrast, (e) upregulation of H3ac is mediated by SB but not SKF. (f) H3p-ac immunoblots (top) and densitometric quantifications (bottom) from cultured striatal neurons treated with D1 agonist (SKF) and/or the NMDA receptor antagonist MK801 and the PKA antagonist H89 as indicated. Notice attenuated response to D1 agonist in the presence of MK801 or H89. (a–f) *p<0.05; **p<0.01.
To confirm the role of D1 receptors for these dynamic changes in H3S10pK14ac for a second rodent species, and to test if functional brain circuitry is required, we used dissociated rat striatal neuronal cultures that lack midbrain and cortical presynaptic inputs. Treatment of striatal cultures with the D1 agonist, SKF82958 (25–50 μM), induced a significant 1.4- to 2.0-fold increase in H3pS10acK14 within 30 min (not shown) and peaked at 3 h (Figure 4d and f; *p<0.05). These changes were specific, because levels of global H3 acetylation remained unaffected in cultures treated with SKF single drug (Figure 4e). In contrast, addition of >100 μM SB to the cell culture medium resulted in significant increases in H3 acetylation and phosphoacetylation (Figure 4d and e).
Finally, given that dopamine-regulated gene expression in striatal neurons involves cyclic AMP and NMDA receptor pathways (Konradi et al, 1996), we examined the effects of NMDA receptor blockade (MK-801, 2 μM) and inhibition of cAMP-dependent protein kinase A (H89, 20 μM). Indeed, pretreatment with MK-801 or H89 prevented or attenuated the upregulation of H3pS10acK14 after D1 agonist treatment (Figure 4f). Taken together, both studies in vitro and in vivo demonstrate that activation of dopamine D1 receptors upregulates bulk chromatin levels of H3pS10acK14 in the nuclei of striatal neurons. Furthermore, D1-regulated striatal H3 phosphoacetylation was, at least in vivo, not modulated by concomitant treatment with the HDACi, SB.
DISCUSSION
Several recent studies identified chromatin remodeling in the striatum—including the regulation of histone acetylation—as an important regulator of stimulant-related plasticity and addiction behavior (Kumar et al, 2005; Levine et al, 2005; Kalda et al, 2007; Renthal et al, 2007). The present study extends the findings of these earlier studies in two ways; first, by demonstrating that chromatin remodeling contributes to drug-induced changes in gene expression in other parts of the reward circuitry, including the ventral midbrain at the level of SN/VTA; second, this report underscores the link between D1 dopamine receptor signaling and chromatin-remodeling mechanisms, by showing that treatment with D1 agonist (i) is associated with increased sensitization and reward behavior upon subsequent exposure to cocaine, (ii) induces a generalized, but short-lasting, nucleosomal response, as defined by the phosphoacetylation of histone H3 in bulk chromatin stimuli (Thomson et al, 1999), and (iii) regulates under certain conditions, including drug-induced inhibition of class I/II HDAC, histone acetylation and gene expression in ventral midbrain chromatin.
Our study faces several limitations, including the broad molecular action of SB, a drug considered a prototype for inhibition of class I/II HDACs but which also interferes with the deacetylation of transcription factors, high-mobility group, and other non-histone proteins important for chromatin function (Davie, 2003). Therefore, the chromatin-remodeling complex that mediates the unexpected histone deacetylation at ventral midbrain Bdnf and Th promoters after cotreatment with D1 agonist and SB, in conjunction with an upregulation of corresponding mRNAs—both shown in the present study—remains to be identified. In any case, given that Bdnf has trophic actions on midbrain dopaminergic neurons (Dluzen et al, 2001; Baker et al, 2005), the observed increase in Bdnf expression may relate, directly or indirectly, to the rise in Th mRNA (ventral midbrain) and protein (striatum) in SKF/SB-treated animals. Notably, expression of Bdnf is dynamically regulated in stress-related models (Lippmann et al, 2007) (and references therein) (Berton et al, 2006; Pu et al, 2006). It will be interesting to determine if D1 agonist and HDACi cotreatments affect expression of GDNF and other growth factors important for ventral midbrain and dopaminergic neuron function (Chen et al, 2006). Notably, recent studies showed that the mood stabilizer, sodium valproate, a short-chain fatty acid structurally similar to sodium butyrate, induces Bdnf expression and promoter-associated histone acetylation in prefrontal cortex (Fukumoto et al, 2001; Bredy et al, 2007). Furthermore, both butyrate and valproate positively regulate a number of intracellular effectors of BDNF signaling, including extracellular signal-related kinases pathways and the Ca2+/cAMP-dependent transcription factor CREB, which in turn upregulate Th expression (Shah et al, 2006; Shaltiel et al, 2007). It is possible that transient increases in neurotrophin supply—including those of BDNF—evoke further adaptations in a subset of dopaminergic or other neurons, which then underlie the observed changes in cocaine-related behaviors. This scenario would also explain important differences in the timing of the various, drug-induced adaptations, which is another limitation of the present study. While the dynamic changes in striatal H3 phosphoacetylation, and Th and Bdnf expression in ventral midbrain were measured within minutes or the first hours after drug treatment, behavioral outcomes were monitored up to 6 days after the last treatments with D1 agonist and HDACi.
Remarkably, the upregulation of striatal histone H3 phosphoacetylation after activation of D1 signaling (present study) or blockade of D2-like signaling (Li et al, 2004) is readily apparent on immunoblots from tissue homogenates or extracts, indicating that this is a ‘nucleosomal response’ (Thomson et al, 1999) that includes, but is not limited to, c-fos, c-jun, and other immediate-early gene promoters (Thomson et al, 1999; Li et al, 2004). In this context, it is noteworthy that D1 agonist-induced H3 phosphoacetylation in striatal neurons is completely blocked by the PKA inhibitor, H89 (Figure 4f). Furthermore, H89 inhibits the nucleosomal response in fibroblasts, via functional inactivation of mitogen- and stress-activated protein kinase 1 (MSK1) (Thomson et al, 1999). Notably, recent reports attributed to MSK1 a key regulatory role for hippocampal and striatal histone acetylation and phosphorylation (Brami-Cherrier et al, 2005; Chwang et al, 2007).
The present paper reports that in animals cotreated with D1 agonist and class I/II HDACi, cocaine-induced reward behavior is enhanced. It is therefore tempting to speculate that the opposite strategy, that is treatment with D1 antagonist and a histone acetyl-transferase inhibitor (HATi), could perhaps prevent, or attenuate, some of the behaviors associated with cocaine reward. Evidence from clinical trials suggest that administration of D1 antagonist drugs to active users may be ineffective or even counterproductive (Haney et al, 2001), albeit reduction of cocaine-induced euphoria and stimulation had also been reported (Romach et al, 1999). However, there is evidence from animal studies that D1 antagonism may prevent relapse (Alleweireldt et al, 2002; Anderson et al, 2003; Sanchez et al, 2003). From the viewpoint of translational medicine, it will therefore be of considerable interest to explore whether or not cotreatment of animals with histone-modifying drugs, including HATi or HDACi, alters reinstatement or relapse of cocaine-related behaviors. Finally, it will be of importance to evaluate how chromatin-modifying drugs would affect the developing brain that may be particularly vulnerable to stimulant drugs (Schmauss, 2000; Crandall et al, 2004).
References
Alleweireldt AT, Weber SM, Kirschner KF, Bullock BL, Neisewander JL (2002). Blockade or stimulation of D1 dopamine receptors attenuates cue reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 159: 284–293.
Anderson SM, Bari AA, Pierce RC (2003). Administration of the D1-like dopamine receptor antagonist SCH-23390 into the medial nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharmacology (Berl) 168: 132–138.
Baker SA, Stanford LE, Brown RE, Hagg T (2005). Maturation but not survival of dopaminergic nigrostriatal neurons is affected in developing and aging BDNF-deficient mice. Brain Res 1039: 177–188.
Belin D, Deroche-Gamonet V, Jaber M (2007). Cocaine-induced sensitization is associated with altered dynamics of transcriptional responses of the dopamine transporter, tyrosine hydroxylase, and dopamine D2 receptors in C57Bl/6J mice. Psychopharmacology (Berl) 193: 567–578.
Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ et al (2006). Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311: 864–868.
Brami-Cherrier K, Valjent E, Herve D, Darragh J, Corvol JC, Pages C et al (2005). Parsing molecular and behavioral effects of cocaine in mitogen- and stress-activated protein kinase-1-deficient mice. J Neurosci 25: 11444–11454.
Bredy TW, Wu H, Crego C, Zellhoefer J, Sun YE, Barad M (2007). Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn Mem 14: 268–276.
Butler R, Bates GP (2006). Histone deacetylase inhibitors as therapeutics for polyglutamine disorders. Nat Rev Neurosci 7: 784–796.
Chandramohan Y, Droste SK, Reul JM (2007). Novelty stress induces phospho-acetylation of histone H3 in rat dentate gyrus granule neurons through coincident signalling via the N-methyl-D-aspartate receptor and the glucocorticoid receptor: relevance for c-fos induction. J Neurochem 101: 815–828.
Chen B, Cepko CL (2007). Requirement of histone deacetylase activity for the expression of critical photoreceptor genes. BMC Dev Biol 7: 78.
Chen PS, Peng GS, Li G, Yang S, Wu X, Wang CC et al (2006). Valproate protects dopaminergic neurons in midbrain neuron/glia cultures by stimulating the release of neurotrophic factors from astrocytes. Mol Psychiatry 11: 1116–1125.
Chwang WB, Arthur JS, Schumacher A, Sweatt JD (2007). The nuclear kinase mitogen- and stress-activated protein kinase 1 regulates hippocampal chromatin remodeling in memory formation. J Neurosci 27: 12732–12742.
Corominas M, Roncero C, Ribases M, Castells X, Casas M (2007). Brain-derived neurotrophic factor and its intracellular signaling pathways in cocaine addiction. Neuropsychobiology 55: 2–13.
Crandall JE, Hackett HE, Tobet SA, Kosofsky BE, Bhide PG (2004). Cocaine exposure decreases GABA neuron migration from the ganglionic eminence to the cerebral cortex in embryonic mice. Cereb Cortex 14: 665–675.
Crosio C, Heitz E, Allis CD, Borrelli E, Sassone-Corsi P (2003). Chromatin remodeling and neuronal response: multiple signaling pathways induce specific histone H3 modifications and early gene expression in hippocampal neurons. J Cell Sci 116: 4905–4914.
Davie JR (2003). Inhibition of histone deacetylase activity by butyrate. J Nutr 133: 2485S–2493S.
De Vries TJ, Cools AR, Shippenberg TS (1998). Infusion of a D-1 receptor agonist into the nucleus accumbens enhances cocaine-induced behavioural sensitization. NeuroReport 9: 1763–1768.
Dehm SM, Hilton TL, Wang EH, Bonham K (2004). SRC proximal and core promoter elements dictate TAF1 dependence and transcriptional repression by histone deacetylase inhibitors. Mol Cell Biol 24: 2296–2307.
Dias BG, Banerjee SB, Duman RS, Vaidya VA (2003). Differential regulation of brain derived neurotrophic factor transcripts by antidepressant treatments in the adult rat brain. Neuropharmacology 45: 553–563.
Dluzen DE, Gao X, Story GM, Anderson LI, Kucera J, Walro JM (2001). Evaluation of nigrostriatal dopaminergic function in adult +/+ and +/− BDNF mutant mice. Exp Neurol 170: 121–128.
Fukumoto T, Morinobu S, Okamoto Y, Kagaya A, Yamawaki S (2001). Chronic lithium treatment increases the expression of brain-derived neurotrophic factor in the rat brain. Psychopharmacology (Berl) 158: 100–106.
Graham DL, Hoppenot R, Hendryx A, Self DW (2007). Differential ability of D1 and D2 dopamine receptor agonists to induce and modulate expression and reinstatement of cocaine place preference in rats. Psychopharmacology (Berl) 191: 719–730.
Graybiel AM, Moratalla R, Robertson HA (1990). Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci USA 87: 6912–6916.
Guillin O, Diaz J, Carroll P, Griffon N, Schwartz JC, Sokoloff P (2001). BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature 411: 86–89.
Hall FS, Drgonova J, Goeb M, Uhl GR (2003). Reduced behavioral effects of cocaine in heterozygous brain-derived neurotrophic factor (BDNF) knockout mice. Neuropsychopharmacology 28: 1485–1490.
Haney M, Ward AS, Foltin RW, Fischman MW (2001). Effects of ecopipam, a selective dopamine D1 antagonist, on smoked cocaine self-administration by humans. Psychopharmacology (Berl) 155: 330–337.
Huang HS, Matevossian A, Jiang Y, Akbarian S (2006). Chromatin immunoprecipitation in postmortem brain. J Neurosci Methods 156: 284–292.
Hummel M, Unterwald EM (2002). D1 dopamine receptor: a putative neurochemical and behavioral link to cocaine action. J Cell Physiol 191: 17–27.
Kalda A, Heidmets LT, Shen HY, Zharkovsky A, Chen JF (2007). Histone deacetylase inhibitors modulates the induction and expression of amphetamine-induced behavioral sensitization partially through an associated learning of the environment in mice. Behav Brain Res 181: 76–84.
Kalivas PW, Volkow ND (2005). The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 162: 1403–1413.
Konradi C, Leveque JC, Hyman SE (1996). Amphetamine and dopamine-induced immediate early gene expression in striatal neurons depends on postsynaptic NMDA receptors and calcium. J Neurosci 16: 4231–4239.
Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT et al (2005). Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron 48: 303–314.
Levine AA, Guan Z, Barco A, Xu S, Kandel ER, Schwartz JH (2005). CREB-binding protein controls response to cocaine by acetylating histones at the fosB promoter in the mouse striatum. Proc Natl Acad Sci USA 102: 19186–19191.
Li J, Guo Y, Schroeder FA, Youngs RM, Schmidt TW, Ferris C et al (2004). Dopamine D2-like antagonists induce chromatin remodeling in striatal neurons through cyclic AMP-protein kinase A and NMDA receptor signaling. J Neurochem 90: 1117–1131.
Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM (2007). Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur J Neurosci 25: 3091–3098.
Mariadason JM, Corner GA, Augenlicht LH (2000). Genetic reprogramming in pathways of colonic cell maturation induced by short chain fatty acids: comparison with trichostatin A, sulindac, and curcumin and implications for chemoprevention of colon cancer. Cancer Res 60: 4561–4572.
Moy LY, Tsai LH (2004). Cyclin-dependent kinase 5 phosphorylates serine 31 of tyrosine hydroxylase and regulates its stability. J Biol Chem 279: 54487–54493.
Nusinzon I, Horvath CM (2005). Histone deacetylases as transcriptional activators? Role reversal in inducible gene regulation. Sci STKE 2005: re11.
Paxinos G, Franklin K (2001). The Mouse Brain in Stereotaxic Coordinates, 2nd edn. Academic Press: San Diego.
Pierce RC, Born B, Adams M, Kalivas PW (1996). Repeated intra-ventral tegmental area administration of SKF-38393 induces behavioral and neurochemical sensitization to a subsequent cocaine challenge. J Pharmacol Exp Ther 278: 384–392.
Pu L, Liu QS, Poo MM (2006). BDNF-dependent synaptic sensitization in midbrain dopamine neurons after cocaine withdrawal. Nat Neurosci 9: 605–607.
Rada-Iglesias A, Enroth S, Ameur A, Koch CM, Clelland GK, Respuela-Alonso P et al (2007). Butyrate mediates decrease of histone acetylation centered on transcription start sites and down-regulation of associated genes. Genome Res 17: 708–719.
Rajadhyaksha A, Leveque J, Macias W, Barczak A, Konradi C (1998). Molecular components of striatal plasticity: the various routes of cyclic AMP pathways. Dev Neurosci 20: 204–215.
Renthal W, Maze I, Krishnan V, Covington III HE, Xiao G, Kumar A et al (2007). Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron 56: 517–529.
Romach MK, Glue P, Kampman K, Kaplan HL, Somer GR, Poole S et al (1999). Attenuation of the euphoric effects of cocaine by the dopamine D1/D5 antagonist ecopipam (SCH 39166). Arch Gen Psychiatry 56: 1101–1106.
Russo-Neustadt AA, Beard RC, Huang YM, Cotman CW (2000). Physical activity and antidepressant treatment potentiate the expression of specific brain-derived neurotrophic factor transcripts in the rat hippocampus. Neuroscience 101: 305–312.
Sanchez CJ, Bailie TM, Wu WR, Li N, Sorg BA (2003). Manipulation of dopamine d1-like receptor activation in the rat medial prefrontal cortex alters stress- and cocaine-induced reinstatement of conditioned place preference behavior. Neuroscience 119: 497–505.
Santini E, Valjent E, Usiello A, Carta M, Borgkvist A, Girault JA et al (2007). Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in L-DOPA-induced dyskinesia. J Neurosci 27: 6995–7005.
Schmauss C (2000). A single dose of methamphetamine leads to a long term reversal of the blunted dopamine D1 receptor-mediated neocortical c-fos responses in mice deficient for D2 and D3 receptors. J Biol Chem 275: 38944–38948.
Self DW, Choi KH, Simmons D, Walker JR, Smagula CS (2004). Extinction training regulates neuroadaptive responses to withdrawal from chronic cocaine self-administration. Learn Mem 11: 648–657.
Self DW, Stein L (1992). The D1 agonists SKF 82958 and SKF 77434 are self-administered by rats. Brain Res 582: 349–352.
Shah P, Nankova BB, Parab S, La Gamma EF (2006). Short chain fatty acids induce TH gene expression via ERK-dependent phosphorylation of CREB protein. Brain Res 1107: 13–23.
Shaltiel G, Chen G, Manji HK (2007). Neurotrophic signaling cascades in the pathophysiology and treatment of bipolar disorder. Curr Opin Pharmacol 7: 22–26.
Sorg BA, Li N, Wu W, Bailie TM (2004). Activation of dopamine D1 receptors in the medial prefrontal cortex produces bidirectional effects on cocaine-induced locomotor activity in rats: effects of repeated stress. Neuroscience 127: 187–196.
Stadler F, Kolb G, Rubusch L, Baker SP, Jones EG, Akbarian S (2005). Histone methylation at gene promoters is associated with developmental regulation and region-specific expression of ionotropic and metabotropic glutamate receptors in human brain. J Neurochem 94: 324–336.
Thomson S, Clayton AL, Hazzalin CA, Rose S, Barratt MJ, Mahadevan LC (1999). The nucleosomal response associated with immediate-early gene induction is mediated via alternative MAP kinase cascades: MSK1 as a potential histone H3/HMG-14 kinase. EMBO J 18: 4779–4793.
Timmusk T, Palm K, Metsis M, Reintam T, Paalme V, Saarma M et al (1993). Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron 10: 475–489.
Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ (2006). Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci 9: 519–525.
Winder DG, Egli RE, Schramm NL, Matthews RT (2002). Synaptic plasticity in drug reward circuitry. Curr Mol Med 2: 667–676.
Acknowledgements
This work was supported by a grant from the National Institutes of Drug Abuse (NIDA) to SA (R01 DA017660).
Author information
Authors and Affiliations
Corresponding author
Additional information
DISCLOSURE/CONFLICT OF INTEREST
The authors of this manuscript have no related financial interests or considerations to disclose.
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Rights and permissions
About this article
Cite this article
Schroeder, F., Penta, K., Matevossian, A. et al. Drug-Induced Activation of Dopamine D1 Receptor Signaling and Inhibition of Class I/II Histone Deacetylase Induce Chromatin Remodeling in Reward Circuitry and Modulate Cocaine-Related Behaviors. Neuropsychopharmacol 33, 2981–2992 (2008). https://doi.org/10.1038/npp.2008.15
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2008.15
Keywords
This article is cited by
-
Methamphetamine-induced region-specific transcriptomic and epigenetic changes in the brain of male rats
Communications Biology (2023)
-
Involvement of the dopaminergic system in the reward-related behavior of pregabalin
Scientific Reports (2021)
-
BDNF rescues BAF53b-dependent synaptic plasticity and cocaine-associated memory in the nucleus accumbens
Nature Communications (2016)
-
Transgenerational Inheritance of Paternal Neurobehavioral Phenotypes: Stress, Addiction, Ageing and Metabolism
Molecular Neurobiology (2016)
-
Epigenetics of Stress, Addiction, and Resilience: Therapeutic Implications
Molecular Neurobiology (2016)