Abstract
While bupropion HCl and practical group counseling (PGC) are commonly used treatments for tobacco dependence, the effects of these treatments on brain function are not well established. For this study, 54 tobacco-dependent cigarette smokers underwent resting 18F-fluorodeoxyglucose–positron emission tomography (FDG–PET) scanning before and after 8 weeks of treatment with bupropion HCl, PGC, or pill placebo. Using Statistical Parametric Mapping (SPM 2), changes in cerebral glucose metabolism from before to after treatment were compared between treatment groups and correlations were determined between amount of daily cigarette usage and cerebral glucose metabolism. Compared with placebo, the two active treatments (bupropion HCl and PGC) had reductions in glucose metabolism in the posterior cingulate gyrus. Further analysis suggested that PGC had a greater effect than bupropion HCl on glucose metabolism in this region. We also found positive correlations between daily cigarette use and glucose metabolism in the left occipital gyrus and parietal–temporal junction. There were no significant negative correlations between daily cigarette use and glucose metabolism. Our findings suggest that bupropion HCl and PGC reduce neural activity much as the performance of a goal-oriented task does in the default mode network of the brain, including the posterior cingulate gyrus. Thus, this study supports the theory that active treatments for tobacco dependence move the brain into a more goal-oriented state.
Similar content being viewed by others
INTRODUCTION
Despite the health consequences of cigarette smoking (CDC, 2004; Jacobs et al, 1999), 20.8% of Americans continue to smoke (CDC, 2007). Although active treatments for tobacco dependence (TD) are more effective than placebo in initiating (Karnath, 2002) and maintaining (Hughes et al, 2007; Stead and Lancaster, 2005) abstinence, only 20–30% of smokers are successful in abstaining after 6–12 months of treatment (Tonnesen et al, 2008). One possible route to the development of more effective treatments for TD is a better understanding of how current treatments, such as bupropion HCl and practical group counseling (PGC) psychotherapy, influence brain activity. We therefore examined the effects of these treatments on resting cerebral glucose metabolism, an index of regional brain function. Because effective treatments reduce cigarette usage, we also examined the effects of reduced cigarette usage on cerebral glucose metabolism.
Bupropion HCl is a first-line treatment for TD that alleviates withdrawal symptoms (Durcan et al, 2002), and is more effective than placebo in maintaining abstinence (Jorenby et al, 1999). The best-established mechanism of action of bupropion HCl is that it blocks the reuptake of dopamine (Balfour, 2001; Holm and Spencer, 2000; Horst and Preskorn, 1998; Stahl et al, 2004). Bupropion HCl has been shown to block the dopamine transporter in the human striatum (Argyelan et al, 2005), though at low levels (Meyer et al, 2002), and has been found to increase whole brain dopamine and norepinephrine in animals (Dhir and Kulkarni, 2007). Bupropion HCl also increases nucleus accumbens dopamine overflow in animals during nicotine withdrawal (Paterson et al, 2007). As for a non-dopaminergic mechanism, bupropion HCl is a noncompetitive antagonist at nicotinic acetylcholine receptors (nAChRs) (Slemmer et al, 2000), and this effect may explain its efficacy in smoking cessation, as nAChRs are the primary target of nicotine (Leshner and Koob, 1999; Tapper et al, 2004). Using 18F-fluorodeoxyglucose–positron emission tomography (FDG–PET), members of our team have also shown that bupropion HCl-treated smokers show less cue-induced activation of anterior cingulate cortex glucose metabolism than untreated smokers (Brody et al, 2004), whereas another group found that bupropion HCl treatment did not affect global glucose metabolism (Nofzinger et al, 2001). Thus, previous research suggests that bupropion HCl treatment attenuates smoking-induced activation in limbic and paralimbic regions, and may produce its effects by increasing levels of monoamines, particularly dopamine, and reducing signaling through nAChRs.
To our knowledge, no previous studies have examined the effects of psychotherapy on cerebral glucose metabolism in smokers. In an FDG–PET study of major depressive disorder (MDD), both cognitive behavioral therapy (CBT) and venlafaxine (a serotonin/norepinephrine reuptake inhibitor) reduced glucose metabolism in the prefrontal cortex; however, CBT reduced, whereas venlafaxine increased glucose metabolism in the posterior cingulate cortex (Kennedy et al, 2007). In a similar study using single photon emission computed tomography, interpersonal therapy (IPT) increased perfusion in the posterior cingulate, venlafaxine increased perfusion in the right posterior temporal cortex, and both treatments increased perfusion in the basal ganglia (Martin et al, 2001). Our group found similar changes in cerebral glucose metabolism after treatment with IPT or paroxetine for MDD, namely decreases in prefrontal and anterior cingulate cortex and increases in left temporal lobe metabolism (Brody et al, 2001). These studies suggest similar changes in brain function accompanying psychotherapy and pharmacotherapy for MDD.
Because smokers who undergo TD treatment typically reduce the number of cigarettes they smoke per day (CPD), the effects of decreased smoking on brain function must also be considered. Acute nicotine administration decreases global glucose metabolism whereas certain regions (eg, medial thalamus, midbrain, and cerebellar vermis) are spared, thus showing relative activation (Stapleton et al, 2003). Other brain regions commonly found to show relative activation following acute nicotine administration include the prefrontal cortex, thalamus, and visual system (Brody, 2006; Domino et al, 2000b; London et al, 1988a). Investigators have also found altered amygdala activity (Rose et al, 2003; Stein et al, 1998) and deactivations of the insula (Domino et al, 2000b). These results imply that smoking results in regional activity changes that are corrected with decreased cigarette usage.
We hypothesized that treatment with bupropion HCl would have similar effects on cerebral glucose metabolism in smokers as patients treated for depression, namely decreases in the cerebellum and anterior cingulate cortex, and that PGC-treated subjects would show similar changes in cerebral glucose metabolism. We further hypothesized that reduced smoking would be correlated with reduced glucose metabolism in regions that previously showed relative activation after acute nicotine administration, namely the prefrontal cortex, thalamus, and occipital cortex/visual system.
MATERIALS AND METHODS
General Study Design
Following an initial anonymous telephone interview, tobacco-dependent cigarette smokers were assessed in-person to verify eligibility. After receiving a description of the study protocol, those who wished to participate signed a consent form approved by the Greater Los Angeles Veterans Affairs Healthcare System institutional review board. Participants then underwent a resting FDG–PET scan, followed within 1 week by a structural magnetic resonance imaging (MRI) scan to aid in localizing brain regions. Participants were randomly assigned to receive smoking cessation treatment with either bupropion HCl, matching pill placebo, or PGC psychotherapy. Following 8 weeks of treatment, subjects underwent another resting FDG–PET scan.
Subjects
Tobacco-dependent cigarette smokers (⩾10 cigarettes/day) were recruited through local newspapers and internet advertisements asking for smokers who were interested in quitting and participating in a brain imaging study. A total of 255 subjects were screened by telephone, and 54 completed the study and had usable data. Nine additional subjects enrolled in the study, but data from these participants were removed because of PET scanning issues (such as excessive movement or PET scanner problems) and subject dropout.
The initial telephone screening collected information about smoking status, medical, substance abuse, and psychiatric histories. Eligible participants were assessed in-person using screening questions from the Structured Clinical Interview for DSM-IV (First et al, 1995). Participants met DSM-IV criteria for nicotine dependence, whereas those meeting criteria for any other axis I disorder (including substance abuse or dependence) were excluded. Subjects currently taking medications that might affect the central nervous system, or with medical histories that might affect an FDG–PET scan (eg, history of significant head trauma or neurological degenerative disorders), were excluded. Subjects who either drank more than the equivalent of 2 cups of coffee per day or experienced caffeine withdrawal symptoms were also excluded. All subjects had a urine drug screen before each scanning session, and women of child-bearing potential had a urine pregnancy test. In addition, pregnant or lactating women and individuals with metal implants were excluded from the study because of the potential risks of radiation exposure and incompatibility with MRI scanning, respectively.
Rating Scales
The Fagerström Test for Nicotine Dependence (FTND) (Fagerstrom, 1978) and the Hamilton Anxiety (HAM-A) (Hamilton, 1969) and Depression (HAM-D) rating scales (Hamilton, 1967), were administered once during each FDG–PET scanning session. To assess cigarette craving, the Urge to Smoke Scale (UTS) (Jarvik et al, 2000) was administered immediately before and after each PET scanning session. We recorded self-reported CPD and obtained exhaled carbon monoxide levels with the Bedfont EC-50 Microsmokerlyzer II (Bedfont Scientific, Williamsburg, VA) before each PET scan as well as during each medication and PGC visit, as measures of recent cigarette usage.
PET Scanning Protocol
Subjects underwent two FDG–PET scans, during which they received 9.1±1.6 (mean±SD) mCi of [18F]fluorodeoxyglucose intravenously, in a room adjacent to the PET scanning room. Subjects then waited in this dimly lit room for the 40-min uptake period of FDG, and were monitored to make sure they were not moving or talking during this time. No cognitive task was administered during the uptake period. PET images were acquired with the GE Advance PET scanner (DeGrado et al, 1994) (General Electric Medical Systems, Milwaukee, WI) with 35 slices in 3-dimensional mode. At the center of the image, axial resolution (in FWHM) is 4.0 mm, transaxial resolution is 3.8 mm, and axial field of view is 15.2 cm. A 1-min scout scan was obtained to aid in positioning within the scanner, followed by a 40-min emission scan. Images were reconstructed using filtered back projection into a 128 × 128 pixel grid. A transmission scan to correct for attenuation was performed before image acquisition using two 68Ge pin sources.
The MRI scan had the following specifications: three-dimensional Fourier-transform (3DFT) spoiled gradient-recalled acquisition with TR=30 ms, TE=7 ms, 30° angle, 2 acquisitions, 256 × 192 view matrix. The acquired volume was reconstructed as roughly 90 contiguous 1.5 mm-thick transaxial slices.
Treatment Protocol
Following the first FDG–PET scan, subjects were randomly assigned (computer-generated list) to treatment with bupropion, matching pill placebo, or practical group counseling. A double-blind paradigm was followed for the bupropion and pill placebo groups. Treatment continued through the second PET scanning session and lasted for 62±2.3 days (mean±SEM). Subjects were instructed to have a target quit date of 2 weeks after initiation of treatment, and were directed not to seek any interventions for smoking cessation outside of the study. All study participants, including those who did not complete the study, were offered free open-label treatment with bupropion, PGC, or both for 2 months following study participation.
The 17 subjects who completed the bupropion part of the study were started on the medication the day following the initial PET scan. As is standard practice (Hays et al, 2001; Jorenby et al, 1999), subjects were started on 150 mg once per day orally, with the dosage increased to 150 mg twice per day on the fourth day. The placebo group consisted of 17 subjects who completed treatment with matching pill placebo, which was film-coated to have the same appearance as the bupropion tablets, and consisted of inert ingredients (methylcellulose, hydroxypropyethycellulose, and hydroxypropylmethylcellulose). Subjects in both the bupropion and placebo groups were treated with the same pill-taking regimen. These participants met with a study physician weekly for 15-minute medication management visits during which each subject's cigarette and medication usage and side effects were monitored, and an exhaled CO level was obtained. No counseling for tobacco-use cessation was provided during these visits to isolate the effects of medication on brain glucose metabolism.
The 20 participants who completed PGC attended twice-weekly hour-long group therapy sessions with a psychotherapist. The general techniques used in the therapy sessions were based on the relapse–prevention model, and included: (1) education about smoking addiction, withdrawal, and relapse; (2) recognizing danger situations that could lead to relapse; (3) developing coping skills, such as avoiding temptation, coping with negative states, reducing overall stress, and distracting attention from smoking urges with other activities; and (4) social support (Carmody, 1990; Fiore et al, 2000). This treatment method has shown superior efficacy to control conditions (Lancaster and Stead, 2000), and approximately the same effectiveness as nicotine replacement therapy (Dooley and Halford, 1992).
Image Analysis
PET image analysis was performed using the software package Statistical Parametric Mapping, version 2 (SPM2; Wellcome Department of Imaging Neuroscience, UK). All PET images were co-registered and spatially normalized to the standardized coordinate system of Montreal Neurological Institute (MNI) space, using the proportional scaling option in SPM2. PET images were smoothed with a 3D-Gaussian smoothing kernel, 10 mm full-width at half-maximum. We measured regional brain radioactivity relative to the global mean value for each subject, which serves as an indicator of relative regional cerebral glucose metabolism, henceforth referred to as glucose metabolism. MRI images of all study subjects were transformed to MNI space and used to create a mean structural image.
Two sets of analyses were performed: (1) a comparison of the effect of treatment on metabolism for the bupropion, PGC, and placebo groups, and (2) an analysis of the effect of CPD on metabolism. Using the Z-statistic on a voxel-by-voxel basis, we identified voxels where the corresponding effect was significant. Only clusters passing the family-wise error (FWE) corrected significance threshold of p<0.05 at either the voxel or cluster level are reported. The anatomical location of each cluster was identified based on the voxel of peak significance.
For the analysis comparing the effect of treatment for the bupropion, PGC, and placebo treatments, the Z-statistic was mapped (an SPM) for the group-by-treatment (pre- and post-treatment) interaction. For these analyses, age, gender, and number of cigarettes per day were entered as covariates of no interest to isolate treatment effects. To identify the group whose data accounted for the significant group-by-treatment interaction, SPMs of the effect of treatment were made for each group separately using the same covariates and statistical thresholds.
For the analysis examining associations between relative brain glucose metabolism and CPD, an analysis of covariance was performed for each voxel, in which conditions were pre- and post-treatment, and the condition-dependent covariate was CPD at the time of scanning. Age, gender, and group were entered as covariates of no interest in this analysis to isolate the effect of CPD. This design allowed us to determine the main effect of CPD and the interaction between effect of treatment and CPD.
Significant clusters were manually localized by a trained rater (MRC) and verified by the laboratory PI (ALB). A mean image of all normalized PET scans used in this study was created using SPM2, and significant clusters were superimposed onto this image. Regions were localized using a standard brain atlas (Mai, 2008). Region locations were also verified using a mean structural MRI image created using SPM2 from the MRI images obtained for this study.
RESULTS
Subject Characteristics and Effects of Treatment
The three treatment groups were similar in age, pre-treatment smoking frequency, number of years smoking, years of education, pre-treatment CPD, and exhaled carbon monoxide levels, (ANOVAs, NS), and in gender and ethnic background distributions (Pearson's χ2-tests, NS) (Table 1). The ethnic makeup of the study sample was 37% White, non-Hispanic, 51% African American, 4% Asian, 2% Hispanic and 6% who self-identified as other or declined to state. The groups were also similar in pre-treatment measures of anxiety (HAM-A), depression (HAM-D) and nicotine dependence (FTND and UTS) (ANOVAs, NS) (Table 1).
After completion of the treatment protocol, six subjects in the PGC group (30%), three subjects in the bupropion group (17%) and two subjects in the placebo group (11%) were abstinent from cigarettes (⩾1 week by self-report and an exhaled CO⩽4 p.p.m. at the second PET session). All subjects included in the analysis maintained compliance with either pill or PGC treatment.
For clinical data, 2-way repeated-measures ANOVAs with interaction between group and effect of treatment were performed, along with post hoc comparisons (t-tests) of the effect of treatment within each group (data in Table 1; statistical results in Table 2). We found highly significant effects of treatment on CPD, exhaled CO, UTS, and FTND, but not on HAM-A or HAM-D (Table 2). CPD decreased significantly in all groups. Exhaled CO decreased in only the bupropion HCl and PGC groups, which were significantly different in this measure from the placebo group. UTS and FTND decreased significantly only in the PGC group.
Cerebral Metabolic Effects of Treatment and Group
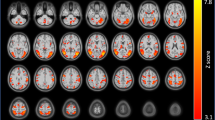
We first examined the SPM for the interaction between effect of treatment and group for a combined ‘active-treatments’ group (including both bupropion HCl- and PGC-treated smokers) vs the placebo-treated group. We found a significant cluster in the posterior cingulate gyrus (p=0.04 corrected, Table 3, Figure 1) corresponding to a decrease in metabolism in the active-treatments group.
Regions of greater decreases in metabolism from pre- to post-treatment in actively treated compared to placebo-treated subjects. The colored region represents an area of significantly greater reduction in metabolism from before to after treatment in subjects given active treatments (bupropion or PGC) compared with placebo (p=0.02, FWE corrected). This region is presented over the T1-weighted MRI template in the Statistical Parametric Mapping (SPM 2) software program. The color bar indicates z-score.
To determine the contribution of each component of the active-treatments group, we examined the interaction between effect of treatment and group for the PGC and bupropion HCl groups separately vs the placebo group. The PGC vs placebo interaction SPM showed a significant cluster in the posterior cingulate gyrus (p=0.01 corrected, Table 3), corresponding to decreased glucose metabolism in the PGC group. The bupropion vs placebo interaction SPM revealed a similar cluster that trended toward but did not reach significance (p=0.07 corrected, Table 3). In neither SPM was there significant clusters corresponding to increased glucose metabolism because of active treatment. There were no significant clusters in the PGC vs bupropion interaction SPM.
As an exploratory analysis of metabolic differences between bupropion HCl and PGC treatment, we examined the PGC vs bupropion HCl interaction SPM using a less stringent statistical threshold (p⩽0.001, uncorrected). There were regions corresponding to decreased glucose metabolism in the PGC group in the medial occipital gyrus, posterior cingulate gyrus extending into the precuneus, and right inferior temporal gyrus. There were regions corresponding to decreased glucose metabolism in the bupropion HCl group in the superior temporal gyrus bilaterally, left amygdala, and anterior cingulate cortex.
To interpret the interaction SPM findings, we examined SPMs for the effect of treatment for the groups separately. Neither the bupropion HCl nor the PGC SPMs showed any clusters of decreased glucose metabolism that met criteria for significance. However, in agreement with the interaction results, there was a cluster showing decreased glucose metabolism in the posterior cingulate gyrus for the PGC group that did not survive correction for multiple comparisons. Increases in glucose metabolism in the bupropion HCl group were observed in the occipital gyrus (p<0.001 corrected, cluster level) and the middle temporal gyrus (p=0.03 corrected, cluster level). For the PGC group, we found increased glucose metabolism in the occipital gyri bilaterally (p<0.001 corrected, cluster level). There were no changes meeting significance criteria in the placebo group.
Effects of the Number of Cigarettes Smoked
The SPM for the main effect of CPD revealed two clusters representing a positive relationship between CPD and glucose metabolism. A large cluster (1745 voxels) in the occipital gyrus and a smaller cluster in the parietal–temporal region (457 voxels) were significant at the voxel level (each p=0.05 corrected). There were no regions representing a negative relationship.
DISCUSSION
Cigarette smokers who completed treatment with either bupropion HCl or PGC showed similar regional changes in relative cerebral glucose metabolism. In the active-treatments group, we found a significant cluster of decreased glucose metabolism in the posterior cingulate gyrus. To determine their relative contributions to the ‘active-treatments’ analysis, we considered the bupropion HCl and PGC groups separately, and (compared with placebo-treated individuals) smokers treated with PGC had a greater reduction in glucose metabolism with treatment in the posterior cingulate gyrus, whereas those treated with bupropion HCl showed a trend towards significance in the same region. Furthermore, these cerebral metabolic changes were not because of the change in number of cigarettes smoked per day (which was controlled for in this statistical analysis). As a direction for future study, we conducted an exploratory analysis to reveal more subtle differences between the active treatments. Our results suggest that effective treatments for cigarette smoking affect regional brain function through similar circuitry.
The posterior cingulate gyrus is a region that shows reduced activity in response to goal-directed tasks, regardless of task type (Gusnard and Raichle, 2001), and is thought to mediate the intrinsic activity of the resting brain as part of the ‘default mode network’ (Fransson and Marrelec, 2008; Raichle et al, 2001). The reduced posterior cingulate gyrus activity found here indicates that treatment for tobacco dependence with bupropion HCl or PGC results in lower resting glucose metabolism in the default mode network. Consistent with our findings, previous studies of bupropion HCl treatment of subjects with MDD showed decreases in activation of the posterior cingulate gyrus and precuneus in response to emotional stimuli (Robertson et al, 2007), perhaps indicating a common mechanism of action when bupropion HCl is used for the treatment of MDD or tobacco dependence.
In the exploratory analysis, comparison between the bupropion HCl and PGC groups revealed differences that were in agreement with the previously mentioned changes, in particular a cluster of greater decrease in the PGC group in the posterior cingulate gyrus. In contrast, the bupropion HCl-treated group showed areas of greater metabolic decreases than the PGC-treated group in several areas including the left amygdala and anterior cingulate cortex, a region we have previously shown to exhibit attenuated cigarette cue-induced activation following bupropion HCl treatment in heavy smokers (Brody et al, 2004). The difference between these active treatments, combined with increased effects at the shared sites (such as the posterior cingulate), may account for the increased efficacy of combined treatment with bupropion HCl and PGC (Reus and Smith, 2008). Owing to the relaxed threshold we used in this comparison (p⩽0.001, uncorrected) these results should be considered preliminary and as providing possible direction for future study.
We found a large cluster representing an area of positive correlation between glucose metabolism and CPD in the posterior occipital gyrus, primarily on the left side but extending to the midline. Increased regional activity in the visual system is often observed in response to acute nicotine administration (Domino et al, 2000a,2000b; London et al, 1988b). Because number of cigarettes per day is correlated with blood nicotine concentration (Hill et al, 1983; Russell et al, 1976) and subjects in our study reduced their cigarette usage (and presumably nicotine intake), our results are consistent with past studies showing a relationship between increased plasma nicotine concentration and increased occipital activity. An additional cluster of positive correlation between daily smoking and regional metabolism was found in the left parietal–temporal junction, extending to the superior temporal gyrus. The superior temporal gyrus is linked to both visual and auditory alertness (Thiel and Fink, 2007), consistent with previous research showing that smoking increases alertness in those who are tobacco dependent (Bates et al, 1995; Thiel and Fink, 2007).
There were several limitations of note in this study. The spatial resolution of the PET scanner may have limited our ability to locate small regions of significant group differences. Before scanning, subjects were allowed to smoke ad libitum, and subjects were scanned in the resting state, which may have resulted in factors outside of the study design, including recent cigarette use, influencing the results. Though we used a specific control for the bupropion group (matching pill placebo), there were no specific control conditions for the many different components of the PGC treatment. Finally, the use of patient report, rather than plasma bupropion or nicotine levels to determine medication compliance and smoking status, respectively, may have limited our ability to assess compliance; however, the use of exhaled carbon monoxide readings and repeated visits with study staff may have minimized this limitation.
The strengths of this study included a relatively large sample size for a study of this type (with 108 PET scans being used for analysis) and the use of standard imaging and image analysis methodology. We used carefully controlled treatment to isolate the effects of bupropion HCl and PGC. It is interesting to note that subjects in the placebo-treated group reduced their reported daily cigarette use, but did not show a corresponding reduction in exhaled CO. This discrepancy may reflect effects of smoking immediately before the post-treatment CO test.
Our results suggest that bupropion HCl and PGC treatment for tobacco dependence have similar effects on regional brain metabolism. Active treatments for tobacco dependence appear to work by lowering resting metabolism in part of the brain default mode network. Thus, this study supports the theory that active treatments for tobacco dependence move the brain into a more goal-oriented state. Future work is needed to assess metabolic changes that occur over a longer-term treatment program, as well as changes that occur when bupropion HCl and PGC are used concurrently (as is commonly done in clinical practice).
References
Argyelan M, Szabo Z, Kanyo B, Tanacs A, Kovacs Z, Janka Z et al (2005). Dopamine transporter availability in medication free and in bupropion treated depression: a 99mTc-TRODAT-1 SPECT study. J Affect Disord 89: 115–123.
Balfour DJ (2001). The pharmacology underlying pharmacotherapy for tobacco dependence: a focus on bupropion. Int J Clin Pract 55: 53–57.
Bates T, Mangan G, Stough C, Corballis P (1995). Smoking, processing speed and attention in a choice reaction time task. Psychopharmacology (Berlin) 120: 209–212.
Brody AL (2006). Functional brain imaging of tobacco use and dependence. J Psychiatr Res 40: 404–418.
Brody AL, Mandelkern MA, Lee G, Smith E, Sadeghi M, Saxena S et al (2004). Attenuation of cue-induced cigarette craving and anterior cingulate cortex activation in bupropion-treated smokers: a preliminary study. Psychiatry Res 130: 269–281.
Brody AL, Saxena S, Stoessel P, Gillies LA, Fairbanks LA, Alborzian S et al (2001). Regional brain metabolic changes in patients with major depression treated with either paroxetine or interpersonal therapy: preliminary findings. Arch Gen Psychiatry 58: 631–640.
Carmody TP (1990). Preventing relapse in the treatment of nicotine addiction: current issues and future directions. J Psychoactive Drugs 22: 211–238.
CDC (2004). 2004 Surgeon General's Report—the health consequences of smoking.
CDC (2007). Cigarette smoking among adults—United States, 2006 In MMWR pp 1157–1161.
DeGrado TR, Turkington TG, Williams JJ, Stearns CW, Hoffman JM, Coleman RE (1994). Performance characteristics of a whole-body PET scanner. J Nucl Med 35: 1398–1406.
Dhir A, Kulkarni SK (2007). Involvement of nitric oxide (NO) signaling pathway in the antidepressant action of bupropion, a dopamine reuptake inhibitor. Eur J Pharmacol 568: 177–185.
Domino EF, Minoshima S, Guthrie S, Ohl L, Ni L, Koeppe RA et al (2000a). Nicotine effects on regional cerebral blood flow in awake, resting tobacco smokers. Synapse 38: 313–321.
Domino EF, Minoshima S, Guthrie SK, Ohl L, Ni L, Koeppe RA et al (2000b). Effects of nicotine on regional cerebral glucose metabolism in awake resting tobacco smokers. Neuroscience 101: 277–282.
Dooley RT, Halford WK (1992). A comparison of relapse prevention with nicotine gum or nicotine fading in modification of smoking. Aust Psychol 27: 186–191.
Durcan MJ, Deener G, White J, Johnston JA, Gonzales D, Niaura R et al (2002). The effect of bupropion sustained-release on cigarette craving after smoking cessation. Clin Ther 24: 540–551.
Fagerstrom KO (1978). Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav 3: 235–241.
Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER et al (2000). Treating Tobacco Use and Dependence. Clinical Practice Guideline. U.S. Department of Health and Human Services. Public Health Service: Rockville, MD.
First MB, Spitzer RL, Gibbon M, Williams JBW (1995). Structured Clinical Interview for DSM-IV Axis I Disorders—Patient Edition (SCID-I/P, version 2.0). Biometrics Research Department, New York State Psychiatric Institute: New York.
Fransson P, Marrelec G (2008). The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: evidence from a partial correlation network analysis. Neuroimage 42: 1178–1184.
Gusnard DA, Raichle ME (2001). Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2: 685–694.
Hamilton M (1967). Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 6: 278–296.
Hamilton M (1969). Diagnosis and rating of anxiety. Br J Psychiatry 3: 76–79.
Hays JT, Hurt RD, Rigotti NA, Niaura R, Gonzales D, Durcan MJ et al (2001). Sustained-release bupropion for pharmacologic relapse prevention after smoking cessation. a randomized, controlled trial. Ann Intern Med 135: 423–433.
Hill P, Haley NJ, Wynder EL (1983). Cigarette smoking: carboxyhemoglobin, plasma nicotine, cotinine and thiocyanate vs self-reported smoking data and cardiovascular disease. J Chronic Dis 36: 439–449.
Holm KJ, Spencer CM (2000). Bupropion: a review of its use in the management of smoking cessation. Drugs 59: 1007–1024.
Horst WD, Preskorn SH (1998). Mechanisms of action and clinical characteristics of three atypical antidepressants: venlafaxine, nefazodone, bupropion. J Affect Disord 51: 237–254.
Hughes JR, Stead LF, Lancaster T (2007). Antidepressants for smoking cessation. Cochrane Database Syst Rev 1: CD000031.
Jacobs Jr DR, Adachi H, Mulder I, Kromhout D, Menotti A, Nissinen A et al (1999). Cigarette smoking and mortality risk: twenty-five-year follow-up of the Seven Countries Study. Arch Intern Med 159: 733–740.
Jarvik ME, Madsen DC, Olmstead RE, Iwamoto-Schaap PN, Elins JL, Benowitz NL (2000). Nicotine blood levels and subjective craving for cigarettes. Pharmacol Biochem Behav 66: 553–558.
Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR et al (1999). A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med 340: 685–691.
Karnath B (2002). Smoking cessation. Am J Med 112: 399–405.
Kennedy SH, Konarski JZ, Segal ZV, Lau MA, Bieling PJ, McIntyre RS et al (2007). Differences in brain glucose metabolism between responders to CBT and venlafaxine in a 16-week randomized controlled trial. Am J Psychiatry 164: 778–788.
Lancaster T, Stead LF (2000). Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev 2: CD001292.
Leshner AI, Koob GF (1999). Drugs of abuse and the brain. Proc Assoc Am Physicians 111: 99–108.
London ED, Connolly RJ, Szikszay M, Wamsley JK, Dam M (1988a). Effects of nicotine on local cerebral glucose utilization in the rat. J Neurosci 8: 3920–3928.
London ED, Dam M, Fanelli RJ (1988b). Nicotine enhances cerebral glucose utilization in central components of the rat visual system. Brain Res Bull 20: 381–385.
Mai JK (2008). Atlas of the Human Brain: 3rd Edition, 3rd edn. Academic Press: New York, NY.
Martin SD, Martin E, Rai SS, Richardson MA, Royall R (2001). Brain blood flow changes in depressed patients treated with interpersonal psychotherapy or venlafaxine hydrochloride: preliminary findings. Arch Gen Psychiatry 58: 641–648.
Meyer JH, Goulding VS, Wilson AA, Hussey D, Christensen BK, Houle S (2002). Bupropion occupancy of the dopamine transporter is low during clinical treatment. Psychopharmacology (Berlin) 163: 102–105.
Nofzinger EA, Berman S, Fasiczka A, Miewald JM, Meltzer CC, Price JC et al (2001). Effects of bupropion SR on anterior paralimbic function during waking and REM sleep in depression: preliminary findings using. Psychiatry Res 106: 95–111.
Paterson NE, Balfour DJ, Markou A (2007). Chronic bupropion attenuated the anhedonic component of nicotine withdrawal in rats via inhibition of dopamine reuptake in the nucleus accumbens shell. Eur J Neurosci 25: 3099–3108.
Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001). A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682.
Reus VI, Smith BJ (2008). Multimodal techniques for smoking cessation: a review of their efficacy and utilisation and clinical practice guidelines. Int J Clin Pract 62: 1753–1768.
Robertson B, Wang L, Diaz MT, Aiello M, Gersing K, Beyer J et al (2007). Effect of bupropion extended release on negative emotion processing in major depressive disorder: a pilot functional magnetic resonance imaging study. J Clin Psychiatry 68: 261–267.
Rose JE, Behm FM, Westman EC, Mathew RJ, London ED, Hawk TC et al (2003). PET studies of the influences of nicotine on neural systems in cigarette smokers. Am J Psychiatry 160: 323–333.
Russell MA, Feyerabend C, Cole PV (1976). Plasma nicotine levels after cigarette smoking and chewing nicotine gum. Br Med J 1: 1043–1046.
Slemmer JE, Martin BR, Damaj MI (2000). Bupropion is a nicotinic antagonist. J Pharmacol Exp Ther 295: 321–327.
Stahl SM, Pradko JF, Haight BR, Modell JG, Rockett CB, Learned-Coughlin S (2004). A review of the neuropharmacology of bupropion, a dual norepinephrine and dopamine reuptake inhibitor. Prim Care Companion J Clin Psychiatry 6: 159–166.
Stapleton JM, Gilson SF, Wong DF, Villemagne VL, Dannals RF, Grayson RF et al (2003). Intravenous nicotine reduces cerebral glucose metabolism: a preliminary study. Neuropsychopharmacology 28: 765–772.
Stead LF, Lancaster T (2005). Group behaviour therapy programmes for smoking cessation. Cochrane Database Syst Rev 2: CD001007.
Stein EA, Pankiewicz J, Harsch HH, Cho JK, Fuller SA, Hoffmann RG et al (1998). Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. Am J Psychiatry 155: 1009–1015.
Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C et al (2004). Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science 306: 1029–1032.
Thiel CM, Fink GR (2007). Visual and auditory alertness: modality-specific and supramodal neural mechanisms and their modulation by nicotine. J Neurophysiol 97: 2758–2768.
Tonnesen P, Mikkelsen K, Bremann L (2008). Smoking cessation with smokeless tobacco and group therapy: an open, randomized, controlled trial. Nicotine Tob Res 10: 1365–1372.
Acknowledgements
Supported by the National Institute on Drug Abuse (ALB (R01 DA20872)), the Veterans Administration (ALB (Merit Review Type I Award)), the Tobacco-Related Disease Research Program (ALB (16RT-0098)), the Office of National Drug Control Policy (EDL (DABT 63-00-C-1003)), and the National Alliance for Research on Schizophrenia and Depression (ALB). Initial study results were presented at the 2008 Society for Research on Nicotine and Tobacco meeting. We thank Josephine Ribe and Michael Clark for technical support in performing positron emission tomography and magnetic resonance imaging scans, respectively.
Author information
Authors and Affiliations
Corresponding author
Additional information
DISCLOSURE
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Costello, M., Mandelkern, M., Shoptaw, S. et al. Effects of Treatment for Tobacco Dependence on Resting Cerebral Glucose Metabolism. Neuropsychopharmacol 35, 605–612 (2010). https://doi.org/10.1038/npp.2009.165
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2009.165
Keywords
This article is cited by
-
Lost in Translation: the Gap Between Neurobiological Mechanisms and Psychosocial Treatment Research for Substance Use Disorders
Current Addiction Reports (2021)
-
Altered spontaneous activity of posterior cingulate cortex and superior temporal gyrus are associated with a smoking cessation treatment outcome using varenicline revealed by regional homogeneity
Brain Imaging and Behavior (2017)
-
Neural correlates of 12-h abstinence-induced craving in young adult smokers: a resting-state study
Brain Imaging and Behavior (2017)
-
Neuroimaging the Effectiveness of Substance Use Disorder Treatments
Journal of Neuroimmune Pharmacology (2016)
-
Altered spontaneous brain activity in heavy smokers revealed by regional homogeneity
Psychopharmacology (2015)