Abstract
Heavy alcohol consumption during young adulthood is a risk factor for the development of serious alcohol use disorders. Research has shown that individual differences in subjective responses to alcohol may affect individuals’ vulnerability to developing alcoholism. Studies comparing the subjective and objective response to alcohol between light and heavy drinkers (HDs), however, have yielded inconsistent results, and neural responses to alcohol in these groups have not been characterized. We performed a double-blind, placebo-controlled, randomized crossover alcohol challenge study comparing functional magnetic resonance imaging and subjective response to intravenously administered 6% v/v ethanol to a target blood alcohol concentration of 0.08% or placebo between HDs and social drinkers (SDs). During the imaging, we presented emotional cues in order to measure how emotion modulated the effects of alcohol on the brain's reward circuitry. We found that, at equivalent blood alcohol concentrations, HDs reported lower subjective alcohol effects than SDs. Alcohol significantly activated the nucleus accumbens in SDs, but not in HDs. Self-reported ratings of intoxication correlated with striatal activation, suggesting that activation may reflect subjective experience of intoxication. Fearful faces significantly activated the amygdala in the SDs only, and this activation was attenuated by alcohol. This study shows that HDs not only experience reduced subjective effects of alcohol, but also demonstrate a blunted response to alcohol in the brain's reward system. Our findings indicate that reduced subjective and neural response to alcohol in HDs may be suggestive of either the development of tolerance to alcohol, or of pre-existing decreased sensitivity to alcohol's effects.
Similar content being viewed by others
INTRODUCTION
Heavy alcohol consumption during young adulthood is a risk factor for the development of serious alcohol use disorders, as well as a potentially hazardous activity in itself. Research has shown that early-onset drinking is associated with lifetime alcohol problems (Chou and Pickering, 1992), and that those who start drinking earlier are more likely to be involved in alcohol-related injuries (Hingson et al, 2000). The biological mechanisms associated with the escalation of heavy drinking, however, are not well understood.
Individual differences in subjective responses to alcohol may affect the individual's vulnerability to developing alcohol use disorders (Schuckit and Duby, 1982; Schuckit, 1984; King et al, 2002; King and Byars, 2004; Schuckit et al, 2009). Some researchers have suggested that individuals who are less sensitive to the effects of alcohol are more vulnerable to developing alcohol dependence (Schuckit, 1994). Differences in the alcohol response between light and heavy drinkers (HDs), however, seem to vary depending on the type of measurement used. In self-report measures of intoxication, for example, HDs report feeling less pronounced alcohol effects than lighter drinkers (Portans et al, 1989; Heath and Martin, 1991; King et al, 2002, 2011; King and Byars, 2004). Other researchers have examined high-risk groups and have found that these individuals have diminished subjective ratings and less pronounced objective responses such as cortisol release or body sway after consuming alcohol (Schuckit, 1984, 1988), but other groups have not found consistent results (Finn and Pihl, 1987; Finn et al, 1990; Stewart et al, 1992; Conrod et al, 1997, 2001). In the current study, we used functional magnetic resonance imaging (fMRI) to measure the neural response to alcohol in light and HDs. To our knowledge, no other studies have examined differences in neural activation in response to alcohol delivery between cohorts with different drinking patterns. Demonstrating differences in brain response to acute alcohol between light and HDs may increase our understanding of the neural mechanisms underlying sensitivity to alcohol.
Most individuals drink alcohol because of its ability to alter emotional states. People typically report that alcohol induces euphoria and relaxation, while reducing stress, tension, and anxiety. Animal studies also suggest that alcohol produces a rewarding as well as an anxiolytic effect (Coop et al, 1990; Blanchard et al, 1993; Spanagel et al, 1995; Da Silva et al, 2005). In a previous study (Gilman et al, 2008), we presented participants with emotional facial images while they received an intravenous alcohol infusion in an fMRI scanner, in order to study how social stimuli interacted with alcohol at the level of regional brain activation. We demonstrated that during a neutral cue presentation (a neutral facial expression), alcohol robustly activated the nucleus accumbens, a brain region associated with both drug and non-drug rewards (Breiter et al, 1997; Stein et al, 1998; Berns et al, 2001; Knutson et al, 2001a, 2001b; O’Doherty et al, 2002; Pagnoni et al, 2002; McClure et al, 2003), but when participants were presented with a negative cue (a fearful facial expression), those structures did not show significant activation. We also demonstrated that alcohol blunted the neural response to fearful faces in the amygdala, a region that preferentially responds to novel, unpleasant or salient stimuli (Garavan et al, 2001; LeDoux, 2003; Fitzgerald et al, 2006). We proposed that alcohol activates regions of the brain associated with reward, and reduces the activation of regions associated with threat or fear.
The goal of the current study was to extend the results of our previous study by comparing the neural response to alcohol among social drinkers (SDs) to the response among HDs, thus revealing differences in brain response associated with heavy alcohol use. Our alcohol clamp methodology (Ramchandani et al, 1999) ensured that every participant achieved the same blood alcohol concentration (BAC) during the study. We predicted, based on the previous literature, that at equivalent BACs, HDs would report reduced subjective responses to alcohol compared with SDs, and would demonstrate less alcohol-induced activation in the striatal reward system. We also predicted that we would observe less alcohol-mediated blunting of fear-related regions in HDs compared with SDs.
MATERIALS AND METHODS
Participants
In all, 14 community-recruited male SDs and 14 non-treatment seeking male HDs participated in this study. HDs were defined as individuals who regularly drank between 20 and 40 standard alcoholic beverages per week, in accordance with the NIAAA definition of risky drinking (NIAAA, 2004). SDs drank 1–14 alcoholic beverages per week. Participants were given the Timeline Followback (TLFB) questionnaire (Sobell et al, 1986) to measure their current drinking history, as well as the Alcohol Use Disorders Identification Test (AUDIT) (Saunders et al, 1993).
Participants underwent a complete medical and psychiatric evaluation, and were excluded if they had an abnormal physical exam or laboratory values outside of normal ranges. They were also excluded if they had never consumed at least two standard drinks of alcohol within 1 h. Participants were right handed.
Participants were given the Structured Clinical Interview for DSM-IV diagnoses (First et al, 2002), and were excluded if they fulfilled the criteria for an axis-I disorder except for an alcohol use disorder. HDs were told of their diagnoses at the end of the study and provided the opportunity to discuss their drinking with a physician. Any participant who expressed an interest in treatment for alcohol problems was referred to treatment resources. All study methods were approved by the NIH Combined Neuroscience Institutional Review Board. All participants were compensated monetarily.
Experimental Procedures
The alcohol infusion procedure has been described previously (Gilman et al, 2008). Alcohol was infused as a 6% v/v solution in saline over 45 min to achieve a target BAC of 0.08 g% in 15 min and maintain that concentration for 30 min. The study consisted of three infusion sessions conducted on separate days.
During the first study session (familiarization session), participants received an alcohol infusion over 45 min while serial breathalyzer measurements were obtained using the Alcotest 7410 handheld breathalyzer (Drager Safety, CO). Subjective response to alcohol was measured using the Biphasic Alcohol Effects Scale (BAES) (Martin et al, 1993) and the modified Drug Effects Questionnaire (DEQ), (de Wit and McCracken, 1990), which were both given before the infusion began and at several time points throughout the infusion. The BAES is a 14-item rating scale that measures alcohol-induced stimulant and sedative effects. The DEQ consists of four questions measuring drug effects that the participants answers on a scale of 0 (not at all) to 5 (extremely). Blood samples (6 ml) were collected before the start of the infusion, and at 15 and 45 min after the start of the infusion. After the infusion was completed, BrAC measurements were taken every 30 min until BrAC fell below 0.02 g% after which participants were sent home.
On the second and third study sessions, participants received the infusions in the fMRI scanner. One of these infusions was saline (placebo) and one was alcohol, given in a double-blind, randomized order. On these days, participants were placed in the scanner following IV catheter insertion. Structural scans were acquired as the infusion began. Target BAC was expected to be achieved at 15 min, and standardized emotional facial expression (EFE) images (Matsumoto, 1988) were presented at 25 min as functional scans were acquired. Participants were instructed to focus on the images, but no response was required. Blood samples (6 ml) were collected before the start of the infusion, 15 min after the start of the infusion, and at 45 min, when the infusion ended. The DEQ was given at baseline, and before and after the presentation of images.
Stimuli
Visual images from a series of EFE images were used in this study. In all, 45 neutral and 45 fearful faces, as well as a non-emotional control cross-hair condition that served as the inter-stimulus interval, were presented in an event-related design that lasted 8 min 30 s. The stimuli were each presented for 2 s, and the ISI ranged from 0 to 8 s. All stimuli were projected onto a screen placed at the foot of the MRI scanner bed and were viewed using a mirror mounted on the head coil.
fMRI Acquisition
Imaging was performed using a 3 T General Electric MRI scanner with a 16-channel head coil. In all, 30 contiguous axial slices were acquired (in-plane resolution 3.75 × 3.75 mm), providing whole-brain coverage. High-resolution structural scans were collected using a T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) pulse sequence, which facilitated localization and co-registration of functional data (matrix 256 × 256 × 124, repetition time (TR)=100 ms, echo time (TE)=12 ms, field of view (FOV)=24 cm). Functional scans were acquired using a T2*-EPIRT sequence that measure changes in blood oxygen level dependent (BOLD) contrast (∼210 volumes, TR=2 s, TE=30 ms, flip angle=90°, matrix 64 × 64, in-plane matrix=128, FOV=24 cm, slice thickness=3.75 mm, 1 mm gap, yielding 3.75 mm cubic voxels).
fMRI Analysis
Analyses focused on changes in BOLD signal contrast that occurred as the participants viewed the images following alcohol or placebo administration. Analyses were conducted using Analysis of Functional Neural Images (AFNI) software (Cox, 1996). Echoplanar image volumes were preprocessed as follows: (1) voxel time series were interpolated to correct for non-simultaneous slice acquisition within each volume (using sinc interpolation and the most inferior slice as a reference). (2) Volumes were corrected for motion in three-dimensional space, and were spatially smoothed using a 6-mm full-width half-maximum smoothing kernel. (3) A mask was created so that all of the background values outside of the brain were set to zero. Statistical maps were generated for each individual separately by linear contrasts between the regressors of interest (neutral and fearful EFE faces). Preprocessed time series data for each individual were then analyzed by multiple regression, which allowed co-variation of variables related to head motion. The regression model consisted of the orthogonal regressors of interest and six regressors of no interest modeling residual motion. Regressors of interest were convolved with a γ-variate function that modeled a hemodynamic response before inclusion in the regression model (Cohen, 1997). Idealized signal time courses were time-locked to image onset.
Anatomical maps of t statistics were normalized by warping to Talairach space and combined into a group map. Next, a statistical map of the main effects of alcohol and facial emotion was computed by performing a voxel-wise ANOVA of the event-related β-coefficients calculated from the general linear model (using inputs of the regression model). In this full, factorial ANOVA, drug (alcohol or placebo) and emotion (fearful or neutral) were within-subject factors, group (HD or SD) was a between-subject factor, and ‘subject’ was a random factor. Next, linear contrasts (voxel-wise t-tests) between the fearful and neutral facial expressions, as well as between the alcohol and placebo condition, were computed between SDs and HDs for each drug condition and emotional stimulus type. These linear contrasts allowed us to directly investigate the effects in which we were interested. A family-wise error rate correction (using a Monte Carlo simulation and the AFNI program 3dClustSim) was applied to rule out false positives. Clusters larger than 40 voxels at a threshold of p<0.01 (with a peak voxel of p<0.001) were considered significant.
T statistics from the group maps were subsequently characterized by assessment of BOLD signal changes in volumes of interest (VOI) of the left nucleus accumbens (−10, 7, −7) and of the right amygdala (26, −8, −15). These VOIs were based on our previous alcohol infusion study (Gilman et al, 2008), as well as on previous literature implicating these regions as active during reward (Knutson et al, 2001a; Matthews et al, 2004; Knutson and Cooper, 2005; Bjork et al, 2007; Mohr et al, 2010). The VOIs were drawn as spheres with a radius of 5 mm. Visual inspection of this mask overlaid atop Talairach-warped structural images indicated that these voxels were localized almost entirely or entirely in striatal gray matter. Signal data were extracted from the time series as follows: (1) signal at each voxel was converted to a deviation from the mean for that voxel across the entire time series, (2) signal was averaged by stimulus type and spatially translated into Talairach space, and (3) a mask was created consisting of the VOI through which each participant's data were extracted. A linear regression was performed between data extracted from the VOI and subjective intoxication measures, in order to assess the functional significance of the activation in this region.
Statistical Analysis of Behavioral Measures
During the familiarization session, participants were given the BAES at the following time points: 0 (immediately before the infusion), 15, 25, 35, 45 (when the infusion ended), and 60 min (15-min post-infusion). Each time point was subtracted from the initial time point (time 0) to get a measure of change from baseline. During the scanning sessions, participants were asked questions from the DEQ at the following time points: 0 (immediately before the infusion), 15, 25 (immediately before the faces were presented), 35 (after the faces were presented), 45 (when the infusion ended), and 60 min (15-min post-infusion). Two-way ANOVAs were performed to investigate main effects of group (HD or SD), time, and their interaction. During the scanning sessions, none of the participants reported any subjective alcohol effects during the placebo infusion. Therefore, analyses were only conducted on data collected during the alcohol session. All statistics were calculated using GraphPad Prism version 5.0 (San Diego, CA, USA). The level of significance (α) was set at 0.05, with Bonferroni's correction for multiple comparisons. For ordinal data (such as intoxication ratings), bivariate correlations were run using Spearman's ρ correlation coefficients.
RESULTS
Subject Characteristics
Characteristics of the two groups are described in Table 1. HDs drank significantly more than SDs, and had higher AUDIT scores. Two HDs were diagnosed with alcohol abuse, and seven were diagnosed with alcohol dependence. The groups did not differ significantly in family history of alcoholism (35.7% of HDs, 14.3% of SDs, χ2=1.17, 1 df, p>0.10). Though there were more cigarette smokers in the HD group (6 out of 14) than the SD group (2 out of 14), this difference was not statistically significant (χ2=2.80, 1 df, p=0.09).
Behavioral Results
During the pre-testing session, HDs reported less stimulation than SDs (F=31.15, p<0.001), but there was no significant effect of time, and no interaction between time and subject group. There was no significant difference between the groups on sedation scores, although there was a modest effect of time (F=2.483, p=0.049), with no interaction (Figure 1a). The BAES was not given during the scan sessions.
Subjective responses to alcohol. (a) Self-reported stimulation during the pre-testing session. SDs reported significantly higher stimulation than HDs; there was no significant difference in sedation. (b) Self-reported drug effects during the fMRI scan. SDs reported significantly higher ratings than HDs. (c) Associations between drinks per week and DEQ responses. SDs are shown in red and HDs in blue.
During the alcohol scanning sessions, there was a significant effect of group (F=27.28, p<0.001) and time (F=8.29, p<0.001) for the question ‘do you feel any drug effects?’ with HDs reporting significant lower scores than SDs (Figure 1b). There was also a significant effect of group (F=35.32, p<0.001) and time (F=6.85, p<0.001) for the question ‘do you feel intoxicated?’ For the question ‘do you like the effects you are feeling?’ there was also an effect of group (F=7.311, p=0.013), though this did not survive multiple comparison corrections. There was no effect of time (F=2.27, p=0.068). SDs and HDs did not show a significant difference in responses to the question ‘would you like more of what you have received?’
After establishing differences between groups, we performed bivariate correlations to examine whether drinking behavior was associated with DEQ responses (Figure 1c). There was a significant negative relationship between drinks per week and the questions ‘do you feel any drug effects?’ (rs=−0.75, p<0.001), ‘do you feel intoxicated?’ (rs=−0.75, p<0.001), and ‘do you like the effects you are feeling?’ (rs=−0.50, p=0.012).
There was no significant difference in the blood alcohol concentrations of SDs (BAC=75.71 mg/ml) and HDs (BAC=78.28 mg/ml) during the alcohol infusions.
Neuroimaging Results
Linear contrasts between alcohol and placebo
During the neutral face presentation, in the SDs, alcohol compared with placebo significantly activated the left nucleus accumbens (NAcc), as well as in the right cingulate gyrus and several temporal regions (Table 2, Figure 2, top panel). In contrast, HDs did not show activation to the alcohol compared withplacebo in these regions; alcohol activated the right middle frontal gyrus and right inferior parietal lobule, and deactivated the right inferior occipital gyrus. A direct comparison between SDs and HDs revealed that SDs demonstrated greater activation than HDs during the alcohol scan bilaterally in the nucleus accumbens, as well as in the right inferior parietal lobule, middle occipital gyrus, and precuneus (Table 3; Figure 2, bottom panel). During the placebo infusion, there were no differences in NAcc activation between the groups.
Top panel: activation to alcohol compared with placebo in SDs. Red-yellow indicates more activation to alcohol than to placebo. HDs (not shown) did not show any differences between activation to alcohol and placebo. Bottom panel: activation to alcohol in HDs compared with SDs. HDs showed less activation than SDs to alcohol (indicated by blue-green) in the bilateral nucleus accumbens. P values are indicated by color bars below the images.
When SDs viewed fearful faces, the alcohol-induced activation was attenuated. There were no significant differences between the alcohol and placebo activation during the viewing of fearful faces in either the SDs or the HDs.
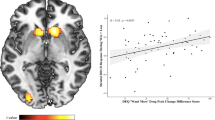
The VOI analysis of the NAcc showed a significant interaction between group (SDs vs HDs) and condition (alcohol vs placebo) (F=3.30, p=0.029). A subsequent t-test showed that SDs had significantly greater NAcc activation than HDs (p=0.009) (Figure 3a). There were no differences in NAcc activation between HDs and SDs during the placebo infusion. NAcc activation was significantly associated with subjective rating of intoxication (rs=0.42, p=0.03) (Figure 3b). We did not find a significant association between NAcc activation and any measures of drinking behavior.
Volume-of-interest analysis of the left NAcc and right amygdala. (a) Interaction between group and alcohol in the Nacc (p=0.029, F=3.30). An asterisk (*) signifies a significant difference (p<0.05) from all other conditions. (b) Association between NAcc activation and subjective rating of intoxication. SDs are shown in red and HDs in blue. (c) Interaction between emotion and alcohol in the amygdala in SDs (left; p=0.024, F=3.34) and HDs (right; p=NS). Please see fMRI analysis methods section for details about VOI data extraction.
Linear contrasts between fearful and neutral faces
In the placebo condition, SDs showed greater activation to the fearful than neutral faces in several frontal, temporal, and visual areas, including the bilateral parahippocampal gyrus and amygdala. When the SDs were given alcohol, there was no significant increase in activation to fearful faces in any brain regions. In the placebo condition, HDs showed greater activation to the fearful than to the neutral faces in the right middle temporal gyrus, and right posterior cingulate. During the alcohol condition, HDs showed greater activation to fearful than neutral faces in the right lingual gyrus and in the bilateral middle frontal gyri (Table 4).
The VOI analysis of the right amygdala showed a significant interaction in SDs between emotion (fearful vs neutral) and alcohol in the amygdala (p=0.024, F=3.34) (Figure 3c, left). Subsequent t-tests showed that SDs demonstrated significantly less activation to fearful faces during the alcohol session than during the placebo session (p=0.018). In HDs, there was no significant interaction between emotion and alcohol (Figure 3c, right).
Factorial ANOVA results
We did not find any significant three-way interactions between alcohol, emotion, and group. We also did not find any interactions between alcohol and emotion, or between emotion and group. There were significant interactions between alcohol and group in the left superior frontal gyrus (F=15.87, p<0.0001), right insula (F=11.54, p<0.0001), left middle frontal gyrus (F=12.41, p<0.0001), and left middle temporal gyrus (F=12.08, p<0.0001).
DISCUSSION
The current study shows that young adult HDs not only experience reduced subjective effects of alcohol, but also demonstrate a blunted response in the brain's reward system. Alcohol significantly activated the NAcc in the SDs, but not the HDs. In both groups, NAcc activation significantly correlated with self-rated intoxication. Fearful faces significantly activated the amygdala and other temporal regions only in SDs; this activation was modulated by alcohol. HDs demonstrated no differences in their response to fearful vs neutral faces during the alcohol or placebo sessions.
HDs Report Reduced Subjective Effects of Alcohol
On the BAES, HDs reported significantly lower scores on stimulation and slightly lower scores on sedation. They also reported reduced DEQ ratings on all questions, except the question ‘Would you like more of what you received?’ This is consistent with previous reports (King et al, 2002) that found HDs reported only slightly higher scores on ‘want more’ while reporting lower scores of ‘feel effects’ and ‘feel high.’ This suggests that HDs may be at particularly high risk for the development for alcohol use disorders, as the desire to continue drinking despite demonstrating reduced positive effects of alcohol can result in consuming greater amounts to reach desired effects.
These differences in self-reported effects of alcohol are indicative of tolerance, defined as the need for increasing amounts of alcohol in order to achieve intoxication or a desired effect, or a markedly diminished effect with continuous use of the same amount of alcohol (APA, 1994). In the HDs, binge drinking, or consumption of multiple drinks in rapid succession, may have led to increased tolerance. Tolerance has been demonstrated among HDs in a variety of self-report studies, which have demonstrated that heavier drinking behavior is correlated with lower subjective responses to alcohol effects (Portans et al, 1989; Heath and Martin, 1991; Ramchandani et al, 2002).
Though acquired tolerance is one explanation for reduced subjective effects in HDs, another possibility is that the decreased effects of alcohol in the HDs may have been pre-existing. Low response to alcohol is thought to be a risk factor for alcoholism, as the need to ingest more drinks to achieve a desired affect leads to heavier drinking patterns among these individuals (Schuckit et al, 2009). This hypothesis suggests that the HDs in this study may drink more heavily because of an inherently lower sensitivity of the brain's reward circuitry to alcohol.
HDs Demonstrate Reduced Activation to Alcohol in the Nucleus Accumbens
Consistent with our previous report (Gilman et al, 2008), the SDs in this study showed greater activation to alcohol than to placebo in several brain regions, including the left nucleus accumbens. In contrast, accumbens activation to alcohol was not seen in the HDs at a BAC of 0.08 g%. There are several explanations for the reduced activation of the NAcc to alcohol in HDs. In vivo microdialysis experiments in rats have shown that oral self-administration of alcohol causes significant increases in extracellular dopamine (DA) levels in the NAcc (Weiss et al, 1993, 1996; Gonzales and Weiss, 1998; Melendez et al, 2002), and that this increase in DA is reduced by repeated alcohol exposure (Smith and Weiss, 1999). Franklin et al (2009) showed that at moderate doses (eg, 1.0 g/kg), there was a significant desensitization of DA D2 receptors following alcohol exposure. In human PET scans, research has shown that alcoholics exhibit a decreased number of DA D2 receptors in the striatum compared with non-alcoholics (Volkow et al, 2009). The reduced NAcc activation that we observe in the HDs could be related to either desensitization or to a reduced number of D2 receptors, as human DA function had been linked to magnitude of the BOLD signal in NAcc (Schott et al, 2008).
The reduced BOLD response in the NAcc may be a cognitive effect as well as a pharmacological effect. Research has suggested that striatal activity may be modulated by cognitive processes. Yoder et al (2009) conducted an experiment with IV alcohol in which they informed participants that they either would or would not receive alcohol. They found alcohol-induced DA release when participants were not expecting alcohol, but not among subjects who expected and then received alcohol (Yoder et al, 2005, 2007). The authors attribute this effect to reward prediction error (Schultz, 2002), which states that DA neurons in the ventral striatum fire when an individual does not expect a reward and one is delivered. Yoder et al suggest that with repeated drinking, the perceived value of alcohol may diminish and not match the expected rewarding effects from earlier drinking experiences. In the real world, HDs may respond by consuming larger quantities of alcohol, but in our controlled experiment, blood alcohol levels were clamped, which may have created a negative prediction error (ie, they expected to feel intoxicated but their BAC was not high enough). The alterations in expectation combined with delivery of alcohol's euphoric effects at increasingly high BACs may drive further heavy drinking behavior.
Although we were able to detect clear differences between SDs and HDs in the NAcc activation to alcohol, the number of drinks per week did not directly correlate with activation in the NAcc. This indicates that the neural response to alcohol is complex, and may be dependent on several factors, such as differences in expectation, personality characteristics, or genetic predisposition, that may not be reflected in a time-line follow back measure of alcohol consumption.
SDs, but not HDs, Show Decreased Amygdala Activity to Fearful Faces
Few neuroimaging studies have examined the effect of acute alcohol on emotional processing. A recent alcohol challenge study also reported reduced amygdala activity to fearful faces under intoxication (Sripada et al, 2011). Most studies of alcohol and emotion, however, have investigated subjective, and not neural, responses to alcohol, and results have been inconclusive. In an experiment in which participants received either alcohol or placebo before viewing negative, positive, and neutral pictures, alcohol diminished the magnitude of the startle response and the skin conductance response regardless of the valence of the stimuli (Stritzke et al, 1995), suggesting that alcohol diminished overall emotional reactivity. In a similar study by Gabel et al (1980), healthy participants viewed negative, positive, and neutral pictures and were then given the choice to drink alcohol. Both negative and positive stimuli resulted in increased arousal, but alcohol consumption was highest after the participants had viewed the positive slides (Gabel et al, 1980). Curtin et al (1998) used the startle reflex as a measure of the effect of alcohol in a stressful situation. Sober and intoxicated individuals were presented with cues that signaled either safety or a threat of electric shock. Autonomic activity, including SCR, heart rate, EMG, and magnitude of startle responses, were all decreased in the intoxicated condition, but no interaction was found between intoxication and threat condition (Curtin et al, 1998). These studies, therefore, have not demonstrated a specific anxiolytic interaction between alcohol and emotional cues, but suggest that alcohol may modulate emotion through ‘dampening’ of emotional reactivity to any emotion. This interpretation could indicate that the modulation of amygdala reactivity to fearful faces in the SDs is not specific to negative emotion. Future studies can present stimuli of different emotional categories (ie, happy, sad, disgust, surprise) to better explain alcohol's influence on different types of emotional processing.
HDs do not Demonstrate Significant Limbic Activity in Response to Fearful Faces
We replicated our earlier results (Gilman et al, 2008) that showed that alcohol modulated brain activity to fearful faces in the amygdala and other frontal and temporal brain regions in SDs. During the placebo condition, fearful compared with neutral faces activated several higher-order visual regions related to emotion in the SDs, in addition to the bilateral amygdala. Other studies have reported similar results (Devinsky et al, 1995; Phillips et al, 2003; Vuilleumier, 2005). In SDs, the increased response to the fearful faces that we observed in the placebo condition was not observed during the alcohol condition, suggesting that alcohol modulated the neural response to a threatening stimulus.
Fearful faces did not activate the amygdala or other temporal regions during either the placebo or alcohol session in HDs. Research has shown that young adults at risk for alcoholism show reduced amygdala activity to fearful faces compared with low-risk subjects (Glahn et al, 2007). These high-risk individuals also show blunted cortisol responses to stress (Sorocco et al, 2006). Hypoactivation of the brain's threat-detection circuitry may confer a greater risk for developing alcoholism, suggesting that deficient amygdala activity may either be a risk factor for, or may be a result of, heavy drinking.
Limitations and Conclusions
This study has several limitations. Our sample size is not large enough to allow us to look for higher-order interactions such as how family history of alcoholism, gender, or personality variables affect the neural response to alcohol. Second, we used only one level of alcohol exposure, which affected both groups differently. Future studies could expose HDs to higher BACs, equating subjective alcohol effects, and investigate whether differences in brain responses remain. Third, we cannot determine if HDs exhibit a reduced response to alcohol as a result of acquired tolerance or pre-existing lower sensitivity. This limitation is present in most studies comparing HDs to controls and is a challenge for future research. In addition, because this was a passive viewing task, there are no measures of task performance, and therefore, we do not know if the SDs were intoxicated to the point of altered perception. Finally, though the groups were not significantly different in smoking status, there were more smokers in the HD group, which could have affected activation of brain reward systems.
A caveat of this analysis is that significant three-way interactions between group, alcohol, and emotion were not observed. There were also no significant interactions between group and emotion, or emotion and alcohol. This negative finding may be a limitation of using factorial ANOVAs with voxel-wise data. Voxel-wise 3-way interactions are often difficult to interpret, and there is no standard approach to correcting for multiple comparisons within a whole-brain ANOVA. Furthermore, data have to meet certain assumptions, such as compound symmetry (equal correlation across levels of a factor) and homoscedasticity (equal variance across the levels of a between-subject factor, such as group) (Brown and Forsythe, 1974), and patient groups often exhibit more variance than controls. In addition, voxel-wise three-way ANOVAs often produce ‘edge’ artifacts with inflated t values. Linear contrasts may offer a solution to directly investigate effects of interest without limitations of compound symmetry, homoscedasticity, and unbalanced designs.
This is the first study to detect a difference in the neural response to alcohol between heavy and SDs, and has clinical implications. Many recovering alcoholics cannot return to moderate levels of drinking, and one explanation is that the brain's reward system may be permanently altered by years of heavy drinking. Another possibility is that these individuals have pre-existing deficiencies in the brain's reward systems, and it therefore will take a greater amount of alcohol for them to achieve the desired effect of the alcohol. This study also has implications for the field of drug abuse, because it suggests that heavy drug users may have lower sensitivity and/or increased tolerance to the drug of abuse, which would make characterization of drug effects in heavy users challenging.
References
Berns GS, McClure SM, Pagnoni G, Montague PR (2001). Predictability modulates human brain response to reward. J Neurosci 21: 2793–2798.
Bjork JM, Smith AR, Danube CL, Hommer DW (2007). Developmental differences in posterior mesofrontal cortex recruitment by risky rewards. J Neurosci 27: 4839–4849.
Blanchard RJ, Magee L, Veniegas R, Blanchard DC (1993). Alcohol and anxiety: ethopharmacological approaches. Prog Neuropsychopharmacol Biol Psychiatry 17: 171–182.
Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD et al (1997). Acute effects of cocaine on human brain activity and emotion. Neuron 19: 591–611.
Brown MB, Forsythe AB (1974). The ANOVA and multiple comparisons for data with heterogeneous variances. Biometrics 30: 719–724.
Chou SP, Pickering RP (1992). Early onset of drinking as a risk factor for lifetime alcohol-related problems. Br J Addict 87: 1199–1204.
Cohen MS (1997). Parametric analysis of fMRI data using linear systems methods. Neuroimage 6: 93–103.
Conrod PJ, Peterson JB, Pihl RO (2001). Reliability and validity of alcohol-induced heart rate increase as a measure of sensitivity to the stimulant properties of alcohol. Psychopharmacology (Berl) 157: 20–30.
Conrod PJ, Peterson JB, Pihl RO, Mankowski S (1997). Biphasic effects of alcohol on heart rate are influenced by alcoholic family history and rate of alcohol ingestion. Alcohol Clin Exp Res 21: 140–149.
Coop CF, McNaughton N, Warnock K, Laverty R (1990). Effects of ethanol and Ro 15-4513 in an electrophysiological model of anxiolytic action. Neuroscience 35: 669–674.
Cox RW (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29: 162–173.
Curtin JJ, Lang AR, Patrick CJ, Stritzke WG (1998). Alcohol and fear-potentiated startle: the role of competing cognitive demands in the stress-reducing effects of intoxication. J Abnorm Psychol 107: 547–557.
Da Silva GE, Vendruscolo LF, Takahashi RN (2005). Effects of ethanol on locomotor and anxiety-like behaviors and the acquisition of ethanol intake in Lewis and spontaneously hypertensive rats. Life Sci 77: 693–706.
de Wit H, McCracken SG (1990). Ethanol self-administration in males with and without an alcoholic first-degree relative. Alcohol Clin Exp Res 14: 63–70.
Devinsky O, Morrell MJ, Vogt BA (1995). Contributions of anterior cingulate cortex to behaviour. Brain 118 (Part 1): 279–306.
Finn PR, Pihl RO (1987). Men at high risk for alcoholism: the effect of alcohol on cardiovascular response to unavoidable shock. J Abnorm Psychol 96: 230–236.
Finn PR, Zeitouni NC, Pihl RO (1990). Effects of alcohol on psychophysiological hyperreactivity to nonaversive and aversive stimuli in men at high risk for alcoholism. J Abnorm Psychol 99: 79–85.
First MB, Spitzer RL, Robert L, Gibbon M, Williams JBL, Janet BW (2002). Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). Biometrics Research, New York State Psychiatric Institute: New York.
Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL (2006). Beyond threat: amygdala reactivity across multiple expressions of facial affect. Neuroimage 30: 1441–1448.
Franklin KM, Engleman EA, Ingraham CM, McClaren JA, Keith CM, McBride WJ et al (2009). A single, moderate ethanol exposure alters extracellular dopamine levels and dopamine d receptor function in the nucleus accumbens of wistar rats. Alcohol Clin Exp Res 33: 1721–1730.
Gabel PC, Noel NE, Keane TM, Lisman SA (1980). Effects of sexual versus fear arousal on alcohol consumption in college males. Behav Res Ther 18: 519–526.
Garavan H, Pendergrass JC, Ross TJ, Stein EA, Risinger RC (2001). Amygdala response to both positively and negatively valenced stimuli. Neuroreport 12: 2779–2783.
Gilman JM, Ramchandani VA, Davis MB, Bjork JM, Hommer DW (2008). Why we like to drink: a functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. J Neurosci 28: 4583–4591.
Glahn DC, Lovallo WR, Fox PT (2007). Reduced amygdala activation in young adults at high risk of alcoholism: studies from the Oklahoma family health patterns project. Biol Psychiatry 61: 1306–1309.
Gonzales RA, Weiss F (1998). Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci 18: 10663–10671.
Heath AC, Martin NG (1991). Intoxication after an acute dose of alcohol: an assessment of its association with alcohol consumption patterns by using twin data. Alcohol Clin Exp Res 15: 122–128.
Hingson RW, Heeren T, Jamanka A, Howland J (2000). Age of drinking onset and unintentional injury involvement after drinking. JAMA 284: 1527–1533.
King AC, Byars JA (2004). Alcohol-induced performance impairment in heavy episodic and light social drinkers. J Stud Alcohol 65: 27–36.
King AC, de Wit H, McNamara PJ, Cao D (2011). Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry 68: 389–399.
King AC, Houle T, de Wit H, Holdstock L, Schuster A (2002). Biphasic alcohol response differs in heavy versus light drinkers. Alcohol Clin Exp Res 26: 827–835.
Knutson B, Adams CM, Fong GW, Hommer D (2001a). Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 21: RC159.
Knutson B, Cooper JC (2005). Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol 18: 411–417.
Knutson B, Fong GW, Adams CM, Varner JL, Hommer D (2001b). Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport 12: 3683–3687.
LeDoux J (2003). The emotional brain, fear, and the amygdala. Cell Mol Neurobiol 23: 727–738.
Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM (1993). Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res 17: 140–146.
Matsumoto D (1988). Japanese and Caucasian Facial Expressions of Emotion and Neutral Faces. San Francisco State University: San Francisco.
Matthews SC, Simmons AN, Lane SD, Paulus MP (2004). Selective activation of the nucleus accumbens during risk-taking decision making. Neuroreport 15: 2123–2127.
McClure SM, Daw ND, Montague PR (2003). A computational substrate for incentive salience. Trends Neurosci 26: 423–428.
Melendez RI, Rodd-Henricks ZA, Engleman EA, Li TK, McBride WJ, Murphy JM (2002). Microdialysis of dopamine in the nucleus accumbens of alcohol-preferring (P) rats during anticipation and operant self-administration of ethanol. Alcohol Clin Exp Res 26: 318–325.
Mohr PN, Biele G, Heekeren HR (2010). Neural processing of risk. J Neurosci 30: 6613–6619.
NIAAA (2004). NIAAA Council Approves Definition of Binge Drinking; WINTER 2004 Number 3, http://pubs.niaaa.nih.gov/publications/Newsletter/winter2004/Newsletter_Number3.pdf.
O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ (2002). Neural responses during anticipation of a primary taste reward. Neuron 33: 815–826.
Pagnoni G, Zink CF, Montague PR, Berns GS (2002). Activity in human ventral striatum locked to errors of reward prediction. Nat Neurosci 5: 97–98.
Phillips ML, Drevets WC, Rauch SL, Lane R (2003). Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry 54: 504–514.
Portans I, White JM, Staiger PK (1989). Acute tolerance to alcohol: changes in subjective effects among social drinkers. Psychopharmacology (Berl) 97: 365–369.
Ramchandani VA, Bolane J, Li TK, O’Connor S (1999). A physiologically-based pharmacokinetic (PBPK) model for alcohol facilitates rapid BrAC clamping. Alcohol Clin Exp Res 23: 617–623.
Ramchandani VA, Flury L, Morzorati SL, Kareken D, Blekher T, Foroud T et al (2002). Recent drinking history: association with family history of alcoholism and the acute response to alcohol during a 60 mg% clamp. J Stud Alcohol 63: 734–744.
Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M (1993). Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption--II. Addiction 88: 791–804.
Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH et al (2008). Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J Neurosci 28: 14311–14319.
Schuckit MA (1984). Subjective responses to alcohol in sons of alcoholics and control subjects. Arch Gen Psychiatry 41: 879–884.
Schuckit MA (1988). Reactions to alcohol in sons of alcoholics and controls. Alcohol Clin Exp Res 12: 465–470.
Schuckit MA (1994). Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry 151: 184–189.
Schuckit MA, Duby J (1982). Alcohol-related flushing and the risk for alcoholism in sons of alcoholics. J Clin Psychiatry 43: 415–418.
Schuckit MA, Smith TL, Danko GP, Trim R, Bucholz KK, Edenberg HJ et al (2009). An evaluation of the full level of response to alcohol model of heavy drinking and problems in COGA offspring. J Stud Alcohol Drugs 70: 436–445.
Schultz W (2002). Getting formal with dopamine and reward. Neuron 36: 241–263.
Smith AD, Weiss F (1999). Ethanol exposure differentially alters central monoamine neurotransmission in alcohol-preferring versus -nonpreferring rats. J Pharmacol Exp Ther 288: 1223–1228.
Sobell MB, Sobell LC, Klajner F, Pavan D, Basian E (1986). The reliability of a timeline method for assessing normal drinker college students’ recent drinking history: utility for alcohol research. Addict Behav 11: 149–161.
Sorocco KH, Lovallo WR, Vincent AS, Collins FL (2006). Blunted hypothalamic-pituitary-adrenocortical axis responsivity to stress in persons with a family history of alcoholism. Int J Psychophysiol 59: 210–217.
Spanagel R, Montkowski A, Allingham K, Stohr T, Shoaib M, Holsboer F et al (1995). Anxiety: a potential predictor of vulnerability to the initiation of ethanol self-administration in rats. Psychopharmacology (Berl) 122: 369–373.
Sripada CS, Angstadt M, McNamara P, King AC, Phan KL (2011). Effects of alcohol on brain responses to social signals of threat in humans. Neuroimage 55: 371–380.
Stein EA, Pankiewicz J, Harsch HH, Cho JK, Fuller SA, Hoffmann RG et al (1998). Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. Am J Psychiatry 155: 1009–1015.
Stewart SH, Finn PR, Pihl RO (1992). The effects of alcohol on the cardiovascular stress response in men at high risk for alcoholism: a dose response study. J Stud Alcohol 53: 499–506.
Stritzke WG, Patrick CJ, Lang AR (1995). Alcohol and human emotion: a multidimensional analysis incorporating startle-probe methodology. J Abnorm Psychol 104: 114–122.
Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F (2009). Imaging dopamine's role in drug abuse and addiction. Neuropharmacology 56 (Suppl 1): 3–8.
Vuilleumier P (2005). How brains beware: neural mechanisms of emotional attention. Trends Cogn Sci 9: 585–594.
Weiss F, Lorang MT, Bloom FE, Koob GF (1993). Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther 267: 250–258.
Weiss F, Parsons LH, Schulteis G, Hyytia P, Lorang MT, Bloom FE et al (1996). Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J Neurosci 16: 3474–3485.
Yoder KK, Constantinescu CC, Kareken DA, Normandin MD, Cheng TE, O’Connor SJ et al (2007). Heterogeneous effects of alcohol on dopamine release in the striatum: a PET study. Alcohol Clin Exp Res 31: 965–973.
Yoder KK, Kareken DA, Seyoum RA, O’Connor SJ, Wang C, Zheng QH et al (2005). Dopamine D(2) receptor availability is associated with subjective responses to alcohol. Alcohol Clin Exp Res 29: 965–970.
Yoder KK, Morris ED, Constantinescu CC, Cheng TE, Normandin MD, O’Connor SJ et al (2009). When what you see isn’t what you get: alcohol cues, alcohol administration, prediction error, and human striatal dopamine. Alcohol Clin Exp Res 33: 139–149.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Gilman, J., Ramchandani, V., Crouss, T. et al. Subjective and Neural Responses to Intravenous Alcohol in Young Adults with Light and Heavy Drinking Patterns. Neuropsychopharmacol 37, 467–477 (2012). https://doi.org/10.1038/npp.2011.206
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2011.206
Keywords
This article is cited by
-
Association of cerebellar and pre-motor cortex gray matter density with subjective intoxication and subjective response following acute alcohol intake
Scientific Reports (2023)
-
People who binge drink show neuroendocrine tolerance to alcohol cues that is associated with immediate and future drinking- results from a randomized clinical experiment
Neuropsychopharmacology (2023)
-
Ventral prefrontal network response to alcohol in young adults with bipolar disorder: a within-subject randomized placebo-controlled alcohol administration study
Neuropsychopharmacology (2023)
-
Characterization of DREADD receptor expression and function in rhesus macaques trained to discriminate ethanol
Neuropsychopharmacology (2022)
-
Striatal activation to monetary reward is associated with alcohol reward sensitivity
Neuropsychopharmacology (2021)