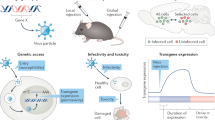
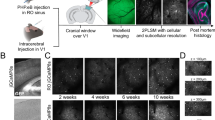
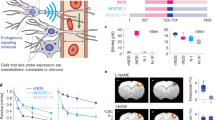
Abstract
Stereotaxic surgery has been an invaluable tool in systems neuroscience, applied in many experiments for the creation of site-targeted lesions, injection of anatomical tracers or implantation of electrodes or microdialysis probes. In this protocol, we describe stereotaxic surgery optimized for gene delivery by recombinant adeno-associated viruses and lentiviruses in mice and rats. This method allows the manipulation of gene expression in the rodent brain with excellent spatiotemporal control; essentially any brain region of choice can be targeted and cells (or a subpopulation of cells) in that region can be stably genetically altered at any postnatal developmental stage up to adulthood. Many aspects of the method, its versatility, ease of application and high reproducibility, make it an attractive approach for studying genetic, cellular and circuit functions in the brain. The entire protocol can be completed in 1–2 hours.
NOTE: In the PDF version of this article initially published online, several lines of text were omitted. The last sentence on page 3167 (“Another well suited application is the use of recombinant viruses for anatomical tracing;”) should have been followed by “for example, simply labeling the entire axonal arborization with GFP27. Finally, in vivo cellular analysis of virally infected and manipulated cortical cells is possible in anesthetized rats and mice with the two-photon targeted-patching technique28–30. As for the study of gene function in behavior, several groups have used viral vectors to infect specific brains regions in mice, such as the midbrain ventral tegmental area and lateral amygdala, and have demonstrated distinct behavioral phenotypes31,32. The main advantage of this approach lies in the exceptional spatiotemporal control over the induced genetic manipulation, which avoids the confounding variables, such as gene alterations during early development and/or in multiple brain regions, typically associated with traditional mouse genetics.” The error has been corrected in the PDF version of the article.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
01 March 2007
uploaded new pdf
References
Deglon, N. & Aebischer, P. Lentiviruses as vectors for CNS diseases. Curr. Top. Microbiol. Immunol. 261, 191–209 (2002).
Tenenbaum, L. et al. Recombinant AAV-mediated gene delivery to the central nervous system. J. Gene Med. 6 Suppl 1, S212–S222 (2004).
Osten, P., Dittgen, T. & Licznerski, P. in The Dynamic Synapse: Molecular Methods in Ionotropic Receptor Biology (eds. Moss, S. & Kittler, J.) 249–259 (CRC, Boca Raton, 2006)
Szczesny, G., Veihelmann, A., Massberg, S., Nolte, D. & Messmer, K. Long-term anaesthesia using inhalatory isoflurane in different strains of mice-the haemodynamic effects. Lab. Anim. 38, 64–69 (2004).
Jevtovic-Todorovic, V. et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J. Neurosci. 23, 876–882 (2003).
Phifer, C.B. & Terry, L.M. Use of hypothermia for general anesthesia in preweanling rodents. Physiol. Behav. 38, 887–890 (1986).
Danneman, P.J. & Mandrell, T.D. Evaluation of five agents/methods for anesthesia of neonatal rats. Lab. Anim. Sci. 47, 386–395 (1997).
Büchen-Osmond, C. The Universal Virus Database of the International Committee on Taxonomy of Viruses <http://www.ncbi.nlm.nih.gov/ICTVdb/index.htm> (2001).
Van Harreveld, A. in The Structure and Function of Nervous Tissue (ed. Bourne, G.H.) 447–511 (Academic Press, 1972).
Thorne, R.G. & Nicholson, C. In vivo diffusion analysis with quantum dots and dextrans predicts the width of brain extracellular space. Proc. Natl. Acad. Sci. USA 103, 5567–5572 (2006).
Lieberman, D.M., Laske, D.W., Morrison, P.F., Bankiewicz, K.S. & Oldfield, E.H. Convection-enhanced distribution of large molecules in gray matter during interstitial drug infusion. J. Neurosurg. 82, 1021–1029 (1995).
Cunningham, J. et al. Distribution of AAV-TK following intracranial convection-enhanced delivery into rats. Cell Transplant. 9, 585–594 (2000).
Nguyen, J.B., Sanchez-Pernaute, R., Cunningham, J. & Bankiewicz, K.S. Convection-enhanced delivery of AAV-2 combined with heparin increases TK gene transfer in the rat brain. Neuroreport 12, 1961–1964 (2001).
Mastakov, M.Y., Baer, K., Xu, R., Fitzsimons, H. & During, M.J. Combined injection of rAAV with mannitol enhances gene expression in the rat brain. Mol. Ther. 3, 225–232 (2001).
Xu, R. et al. Quantitative comparison of expression with adeno-associated virus (AAV-2) brain-specific gene cassettes. Gene Ther. 8, 1323–1332 (2001).
Kugler, S., Lingor, P., Scholl, U., Zolotukhin, S. & Bahr, M. Differential transgene expression in brain cells in vivo and in vitro from AAV-2 vectors with small transcriptional control units. Virology 311, 89–95 (2003).
Klugmann, M. et al. AAV-mediated hippocampal expression of short and long Homer 1 proteins differentially affect cognition and seizure activity in adult rats. Mol. Cell. Neurosci. 28, 347–360 (2005).
Callaway, E.M. A molecular and genetic arsenal for systems neuroscience. Trends Neurosci. 28, 196–201 (2005).
Burger, C. et al. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol. Ther. 10, 302–317 (2004).
Davidson, B.L. et al. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc. Natl. Acad. Sci. USA 97, 3428–3432 (2000).
Burns, J.C., Friedmann, T., Driever, W., Burrascano, M. & Yee, J.K. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc. Natl. Acad. Sci. USA 90, 8033–8037 (1993).
Wiznerowicz, M. & Trono, D. Harnessing HIV for therapy, basic research and biotechnology. Trends Biotechnol. 23, 42–47 (2005).
Mazarakis, N.D. et al. Rabies virus glycoprotein pseudotyping of lentiviral vectors enables retrograde axonal transport and access to the nervous system after peripheral delivery. Hum. Mol. Genet. 10, 2109–2121 (2001).
Muller, O.J. et al. Random peptide libraries displayed on adeno-associated virus to select for targeted gene therapy vectors. Nat. Biotechnol. 21, 1040–1046 (2003).
Yang, L., Bailey, L., Baltimore, D. & Wang, P. Targeting lentiviral vectors to specific cell types in vivo. Proc. Natl. Acad. Sci. USA 103, 11479–11484 (2006).
Dittgen, T. et al. Lentivirus-based genetic manipulations of cortical neurons and their optical and electrophysiological monitoring in vivo. Proc. Natl. Acad. Sci. USA 101, 18206–18211 (2004).
Grinevich, V., Brecht, M. & Osten, P. Monosynaptic pathway from rat vibrissa motor cortex to facial motor neurons revealed by lentivirus-based axonal tracing. J. Neurosci. 25, 8250–8258 (2005).
Margrie, T.W. et al. Targeted whole-cell recordings in the mammalian brain in vivo. Neuron 39, 911–918 (2003).
Komai, S., Denk, W., Osten, P., Brecht, M. & Margrie, T.W. Two-photon targeted patching (TPTP) in vivo. Nat. Protoc. 1, 648–653 (2006).
Komai, S. et al. Postsynaptic excitability is necessary for strengthening of cortical sensory responses during experience-dependent development. Nat. Neurosci. 9, 1125–1133 (2006).
Maskos, U. et al. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature 436, 103–107 (2005).
Rumpel, S., LeDoux, J., Zador, A. & Malinow, R. Postsynaptic receptor trafficking underlying a form of associative learning. Science 308, 83–88 (2005).
Davis, J.A. Mouse and rat anesthesia and analgesia in Current Protocols in Neuroscience: Appendix 4 Animal Techniques (eds. Crawley, J.N., Gerfen, C.R., Rogawski, M.A., Sibley, D.R., Skolnick, P. & Wray, S.) A.4B.1–A.4B.17 (John Wiley & Sons, Inc., NY, 2001).
Acknowledgements
We thank M. Brecht, F. Helmchen, T. Margrie and J. Waters for early help with surgical techniques, and J.Kuhl-Osten for figure graphics. This work was supported in part by the Human Frontier Science Program (RGY79/2005 to P.O.), the German-Israeli Foundation for Scientific Research and Development (733-60.13/2002 to P.O.) and the European Commission (EUsynapse project LSHM-CT-2005-019055 to P.H.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Cetin, A., Komai, S., Eliava, M. et al. Stereotaxic gene delivery in the rodent brain. Nat Protoc 1, 3166–3173 (2006). https://doi.org/10.1038/nprot.2006.450
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2006.450
This article is cited by
-
In vivo identification of astrocyte and neuron subproteomes by proximity-dependent biotinylation
Nature Protocols (2024)
-
Treadmill Running Regulates Adult Neurogenesis, Spatial and Non-spatial Learning, Parvalbumin Neuron Activity by ErbB4 Signaling
Cellular and Molecular Neurobiology (2024)
-
Suv39h1 Silencing Recovers Memory Decline in Scopolamine-Induced Amnesic Mouse Model
Molecular Neurobiology (2024)
-
YTHDF2 in dentate gyrus is the m6A reader mediating m6A modification in hippocampus-dependent learning and memory
Molecular Psychiatry (2023)
-
Ablation of Projection Glutamatergic Neurons in the Lateral Cerebellar Nuclei Alters Motor Coordination in Vglut2-Cre+ Mice
The Cerebellum (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.