Abstract
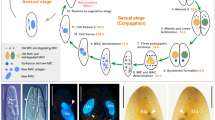
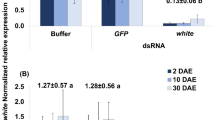
The wasp Nasonia vitripennis is emerging as a useful model organism in which to address a variety of biological questions, due, in part, to its ease of laboratory use, unique aspects of its biology and the sequencing of its genome. In order to take full advantage of the potential of this organism, methods for manipulating gene function are needed. To this end, a protocol for parental RNA interference (pRNAi) in N. vitripennis is described. pRNAi entails injecting pupae with double-stranded RNA, allowing the injected wasps to eclose and examining the progeny for developmental defects. This basic protocol is described in the context of the life cycle of N. vitripennis. This technique has been useful in elucidating the function of most, although not all, genes tested to date, and has potential applications beyond embryonic patterning. pRNAi experiments in Nasonia can be completed in as little as 2 weeks.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
References
Fire, A. et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391, 806–811 (1998).
Lohmann, J.U., Endl, I. & Bosch, T.C. Silencing of developmental genes in hydra. Dev. Biol. 214, 211–214 (1999).
Reddien, P.W., Bermange, A.L., Murfitt, K.J., Jennings, J.R. & Sanchez Alvarado, A. Identification of genes needed for regeneration, stem cell function, and tissue homeostasis by systematic gene perturbation in planaria. Dev. Cell 8, 635–649 (2005).
Bucher, G., Scholten, J. & Klingler, M. Parental RNAi in Tribolium (Coleoptera). Curr. Biol. 12, R85–R86 (2002).
Copf, T., Schroder, R. & Averof, M. Ancestral role of caudal genes in axis elongation and segmentation. Proc. Natl. Acad. Sci. USA 101, 17711–17715 (2004).
Mito, T. et al. Non-canonical functions of hunchback in segment patterning of the intermediate germ cricket Gryllus bimaculatus. Development 132, 2069–2079 (2005).
Liu, P.Z. & Kaufman, T.C. hunchback is required for suppression of abdominal identity, and for proper germband growth and segmentation in the intermediate germband insect Oncopeltus fasciatus. Development 131, 1515–1527 (2004).
Lynch, J.A., Brent, A.E., Leaf, D.S., Pultz, M.A. & Desplan, C. Localized maternal orthodenticle patterns anterior and posterior in the long germ wasp Nasonia. Nature 439, 728–732 (2006).
Whiting, A.R. The biology of the parasitic wasp Mormoniella vitripennis [=Nasonia brevicornis] (Walker). Quart. Rev. Biol. 42, 333–406 (1967).
Azab, A.K., Tawfik, M.F.S. & Awadallah, K.T. Morphology of the early stages of Nasonia vitripennis Walker. Bull. Soc. Ent. Egypte 457, 457–467 (1967).
Schneiderman, H.A. & Horwitz, J. The induction and termination of facultative diapause in the chalcid wasps Mormoniella vitripennis (Walker) and Tritneptis klugii (Ratzeburg). J. Exp. Biol. 35, 520–551 (1958).
Lynch, J.A., Olesnicky, E.C. & Desplan, C. Regulation and function of tailless in the long germ wasp Nasonia vitripennis. Dev. Genes Evol. (2006); published online 3 May (doi:10.1007/s00427-006-0076-5).
Tram, U. & Sullivan, W. Role of delayed nuclear envelope breakdown and mitosis in Wolbachia-induced cytoplasmic incompatibility. Science 296, 1124–1126 (2002).
Ferree, P.M., McDonald, K., Fasulo, B. & Sullivan, W. The origin of centrosomes in parthenogenetic hymenopteran insects. Curr. Biol. 16, 801–807 (2006).
Beukeboom, L.W. & Kamping, A. No patrigenes required for femaleness in the haplodiploid wasp Nasonia vitripennis. Genetics 172, 981–989 (2006).
Trent, C., Crosby, C. & Eavey, J. Additional evidence for the genomic imprinting model of sex determination in the haplodiploid wasp Nasonia vitripennis: isolation of biparental diploid males after X-ray mutagenesis. Heredity 96, 368–376 (2006).
Shuker, D.M. & West, S.A. Information constraints and the precision of adaptation: sex ratio manipulation in wasps. Proc. Natl. Acad. Sci. USA 101, 10363–10367 (2004).
Beukeboom, L.W. & van den Assem, J. Courtship and mating behaviour of interspecific Nasonia hybrids (Hymenoptera, Pteromalidae): a grandfather effect. Behav. Genet. 31, 167–177 (2001).
Bordenstein, S.R., O'Hara, F.P. & Werren, J.H. Wolbachia-induced incompatibility precedes other hybrid incompatibilities in Nasonia. Nature 409, 707–710 (2001).
Gadau, J., Page, R.E. & Werren, J.H. The genetic basis of the interspecific differences in wing size in Nasonia (Hymenoptera; Pteromalidae): major quantitative trait loci and epistasis. Genetics 161, 673–684 (2002).
Pultz, M.A. & Leaf, D.S. The jewel wasp Nasonia: querying the genome with haplo-diploid genetics. Genesis 35, 185–191 (2003).
Pultz, M.A., Pitt, J.N. & Alto, N.M. Extensive zygotic control of the anteroposterior axis in the wasp Nasonia vitripennis. Development 126, 701–710 (1999).
Bull, A.L. Stages of living embryos in the jewel wasp Mormoniella (Nasonia) vitripennis (Walker). Int. J. Insect Morphol. Embryol. 11, 1–23 (1982)
Acknowledgements
We would like to thank Mary Anne Pultz and David Leaf for providing invaluable knowledge and assistance in working with Nasonia. This work was supported by a NIH grant to C.D.; J.A.L. was supported by a NIH Training Grant and a Dean Dissertation Fellowship from NYU. This investigation was conducted in a facility constructed with support from a Research Facilities Improvement grant from NCRR, NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Lynch, J., Desplan, C. A method for parental RNA interference in the wasp Nasonia vitripennis. Nat Protoc 1, 486–494 (2006). https://doi.org/10.1038/nprot.2006.70
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2006.70
This article is cited by
-
Ability of a selfish B chromosome to evade genome elimination in the jewel wasp, Nasonia vitripennis
Heredity (2023)
-
Glucosamine-6-phosphate N-acetyltransferase gene silencing by parental RNA interference in rice leaf folder, Cnaphalocrocis medinalis (Lepidoptera: Pyralidae)
Scientific Reports (2022)
-
RNAi for management of Asian long-horned beetle, Anoplophora glabripennis: identification of target genes
Journal of Pest Science (2020)
-
Ankyrin domain encoding genes from an ancient horizontal transfer are functionally integrated into Nasonia developmental gene regulatory networks
Genome Biology (2018)
-
Plant-mediated RNAi silences midgut-expressed genes in congeneric lepidopteran insects in nature
BMC Plant Biology (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.