Abstract
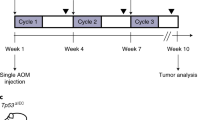
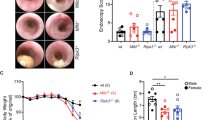
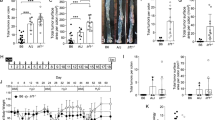
Colorectal cancer is a life-threatening disease that can develop spontaneously or as a complication of inflammatory bowel diseases. Mouse models are essential tools for the preclinical testing of novel therapeutic options in vivo. Here, we provide a highly reliable protocol for an experimental mouse model to study the development of colon cancers. It is based on the mutagenic agent azoxymethane (AOM), which exerts colonotropic carcinogenicity. Repeated intraperitoneal administration of AOM results in the development of spontaneous tumors within 30 weeks. As an alternative option, inflammation-dependent tumor growth can be investigated by combining the administration of AOM with the inflammatory agent dextran sodium sulfate in drinking water, which causes rapid growth of multiple colon tumors per mouse within 10 weeks. Different scoring systems including number of tumors and tumor size identify factors promoting or inhibiting tumor initiation and/or tumor progression, respectively.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Powell, S.M. et al. Molecular diagnosis of familial adenomatous polyposis. N. Engl. J. Med. 329, 1982–1987 (1993).
Corpet, D.E. & Pierre, F. How good are rodent models of carcinogenesis in predicting efficacy in humans? A systematic review and meta-analysis of colon chemoprevention in rats, mice and men. Eur. J. Cancer 41, 1911–1922 (2005).
Moser, A.R., Pitot, H.C. & Dove, W.F. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science 247, 322–324 (1990).
Papanikolaou, A. et al. Initial levels of azoxymethane-induced DNA methyl adducts are not predictive of tumor susceptibility in inbred mice. Toxicol. Appl. Pharmacol. 150, 196–203 (1998).
Evans, J.T., Hauschka, T.S. & Mittelman, A. Differential susceptibility of four mouse strains to induction of multiple large-bowel neoplasms by 1,2-dimethylhydrazine. J. Natl. Cancer Inst. 52, 999–1000 (1974).
Rosenberg, D.W. & Liu, Y. Induction of aberrant crypts in murine colon with varying sensitivity to colon carcinogenesis. Cancer Lett. 92, 209–214 (1995).
Druckrey, E. Production of colonic carcinomas by 1,2–dialkylhydrazines and azoxyalkanes in Carcinoma of the Colon and the Antecedent Epithelium (ed. Burdette, W.J.) 267–279 (Thomas, Springfield, IL, 1970).
Moriya, M., Harada, T. & Shirasu, Y. Inhibition of carcinogenicities of 1,2-dimethylhydrazine and azoxymethane by pyrazole. Cancer Lett. 17, 147–152 (1982).
Bissahoyo, A. et al. Azoxymethane is a genetic background-dependent colorectal tumor initiator and promoter in mice: effects of dose, route, and diet. Toxicol. Sci. 88, 340–345 (2005).
Sohn, O.S., Fiala, E.S., Requeijo, S.P., Weisburger, J.H. & Gonzalez, F.J. Differential effects of CYP2E1 status on the metabolic activation of the colon carcinogens azoxymethane and methylazoxymethanol. Cancer Res. 61, 8435–8440 (2001).
Fiala, E.S. Investigations into the metabolism and mode of action of the colon carcinogens 1,2-dimethylhydrazine and azoxymethane. Cancer 40, 2436–2445 (1977).
Reddy, B.S., Weisburger, J.H., Narisawa, T. & Wynder, E.L. Colon carcinogenesis in germ-free rats with 1,2-dimethylhydrazine and N-methyl-n′-nitro-N-nitrosoguanidine. Cancer Res. 34, 2368–2372 (1974).
Baek, S.J. et al. Nonsteroidal anti-inflammatory drug-activated gene-1 over expression in transgenic mice suppresses intestinal neoplasia. Gastroenterology 131, 1553–1560 (2006).
Becker, C. et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity 21, 491–501 (2004).
Greten, F.R. et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118, 285–296 (2004).
Boivin, G.P. et al. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology 124, 762–777 (2003).
Nambiar, P.R. et al. Preliminary analysis of azoxymethane induced colon tumors in inbred mice commonly used as transgenic/knockout progenitors. Int. J. Oncol. 22, 145–150 (2003).
Shamsuddin, A.M. Comparative studies of primary, metastatic and transplanted colon adenocarcinomas of Fischer 344 rats. J. Submicrosc. Cytol. 16, 327–339 (1984).
Maltzman, T., Whittington, J., Driggers, L., Stephens, J. & Ahnen, D. AOM-induced mouse colon tumors do not express full-length APC protein. Carcinogenesis 18, 2435–2439 (1997).
Takahashi, M., Nakatsugi, S., Sugimura, T. & Wakabayashi, K. Frequent mutations of the beta-catenin gene in mouse colon tumors induced by azoxymethane. Carcinogenesis 21, 1117–1120 (2000).
Guillem, J.G. et al. Changes in expression of oncogenes and endogenous retroviral-like sequences during colon carcinogenesis. Cancer Res. 48, 3964–3971 (1988).
Wang, Q.S., Papanikolaou, A., Sabourin, C.L. & Rosenberg, D.W. Altered expression of cyclin D1 and cyclin-dependent kinase 4 in azoxymethane-induced mouse colon tumorigenesis. Carcinogenesis 19, 2001–2006 (1998).
Vivona, A.A. et al. K-ras mutations in aberrant crypt foci, adenomas and adenocarcinomas during azoxymethane-induced colon carcinogenesis. Carcinogenesis 14, 1777–1781 (1993).
Takahashi, M., Mutoh, M., Kawamori, T., Sugimura, T. & Wakabayashi, K. Altered expression of beta-catenin, inducible nitric oxide synthase and cyclooxygenase-2 in azoxymethane-induced rat colon carcinogenesis. Carcinogenesis 21, 1319–1327 (2000).
Okayasu, I. et al. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 98, 694–702 (1990).
Tanaka, T. et al. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 94, 965–973 (2003).
Suzuki, R., Kohno, H., Sugie, S., Nakagama, H. & Tanaka, T. Strain differences in the susceptibility to azoxymethane and dextran sodium sulfate-induced colon carcinogenesis in mice. Carcinogenesis 27, 162–169 (2006).
Kohno, H. et al. Dietary administration with prenyloxycoumarins, auraptene and collinin, inhibits colitis-related colon carcinogenesis in mice. Int. J. Cancer 118, 2936–2942 (2006).
Okayasu, I., Ohkusa, T., Kajiura, K., Kanno, J. & Sakamoto, S. Promotion of colorectal neoplasia in experimental murine ulcerative colitis. Gut 39, 87–92 (1996).
Becker, C. et al. In vivo imaging of colitis and colon cancer development in mice using high resolution chromoendoscopy. Gut 54, 950–954 (2005).
Papanikolaou, A., Wang, Q.S., Papanikolaou, D., Whiteley, H.E. & Rosenberg, D.W. Sequential and morphological analyses of aberrant crypt foci formation in mice of differing susceptibility to azoxymethane-induced colon carcinogenesis. Carcinogenesis 21, 1567–1572 (2000).
Kumar, S.P., Roy, S.J., Tokumo, K. & Reddy, B.S. Effect of different levels of calorie restriction on azoxymethane-induced colon carcinogenesis in male F344 rats. Cancer Res. 50, 5761–5766 (1990).
Kawamori, T. et al. Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res. 59, 597–601 (1999).
Bird, R.P. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: preliminary findings. Cancer Lett. 37, 147–151 (1987).
Takayama, T. et al. Aberrant crypt foci of the colon as precursors of adenoma and cancer. N. Engl. J. Med. 339, 1277–1284 (1998).
Caderni, G. et al. Identification of mucin-depleted foci in the unsectioned colon of azoxymethane-treated rats: correlation with carcinogenesis. Cancer Res. 63, 2388–2392 (2003).
Kudo, S. et al. Colorectal tumours and pit pattern. J. Clin. Pathol. 47, 880–885 (1994).
Nambiar, P.R. et al. Genetic signatures of high- and low-risk aberrant crypt foci in a mouse model of sporadic colon cancer. Cancer Res. 64, 6394–6401 (2004).
Acknowledgements
We thank Alexei Nikolajew for major contributions regarding animal work and immunohistochemistry. This work was supported by grants from the Deutsche Forschungsgemeinschaft (GK1043 to C.N., C.B. and M.F.N. and SFB 432 to M.F.N.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Neufert, C., Becker, C. & Neurath, M. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat Protoc 2, 1998–2004 (2007). https://doi.org/10.1038/nprot.2007.279
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2007.279
This article is cited by
-
Establishment of a large-scale patient-derived high-risk colorectal adenoma organoid biobank for high-throughput and high-content drug screening
BMC Medicine (2023)
-
Serotonin acts through YAP to promote cell proliferation: mechanism and implication in colorectal cancer progression
Cell Communication and Signaling (2023)
-
Cholesterol reprograms glucose and lipid metabolism to promote proliferation in colon cancer cells
Cancer & Metabolism (2023)
-
Reduced smooth muscle-fibroblasts transformation potentially decreases intestinal wound healing and colitis-associated cancer in ageing mice
Signal Transduction and Targeted Therapy (2023)
-
Tumor-associated macrophages confer colorectal cancer 5-fluorouracil resistance by promoting MRP1 membrane translocation via an intercellular CXCL17/CXCL22–CCR4–ATF6–GRP78 axis
Cell Death & Disease (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.