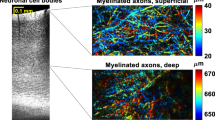
Abstract
Imaging neurons, glia and vasculature in the living brain has become an important experimental tool for understanding how the brain works. Here we describe in detail a protocol for imaging cortical structures at high optical resolution through a thinned-skull cranial window in live mice using two-photon laser scanning microscopy (TPLSM). Surgery can be performed within 30–45 min and images can be acquired immediately thereafter. The procedure can be repeated multiple times allowing longitudinal imaging of the cortex over intervals ranging from days to years. Imaging through a thinned-skull cranial window avoids exposure of the meninges and the cortex, thus providing a minimally invasive approach for studying structural and functional changes of cells under normal and pathological conditions in the living brain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Davalos, D. et al. ATP mediates rapid microglial response to local brain injury in vivo . Nat. Neurosci. 8, 752–758 (2005).
Grutzendler, J., Kasthuri, N. & Gan, W.B. Long-term dendritic spine stability in the adult cortex. Nature 420, 812–816 (2002).
Majewska, A.K., Newton, J.R. & Sur, M. Remodeling of synaptic structure in sensory cortical areas in vivo . J. Neurosci. 26, 3021–3029 (2006).
Nishiyama, H., Fukaya, M., Watanabe, M. & Linden, D.J. Axonal motility and its modulation by activity are branch-type specific in the intact adult cerebellum. Neuron 56, 472–487 (2007).
Stosiek, C., Garaschuk, O., Holthoff, K. & Konnerth, A. In vivo two-photon calcium imaging of neuronal networks. Proc. Natl. Acad. Sci. USA 100, 7319–7324 (2003).
Tsai, J., Grutzendler, J., Duff, K. & Gan, W.B. Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat. Neurosci. 7, 1181–1183 (2004).
Xu, H.T., Pan, F., Yang, G. & Gan, W.B. Choice of cranial window type for in vivo imaging affects dendritic spine turnover in the cortex. Nat. Neurosci. 10, 549–551 (2007).
Yoder, E.J. & Kleinfeld, D. Cortical imaging through the intact mouse skull using two-photon excitation laser scanning microscopy. Microsc. Res. Tech. 56, 304–305 (2002).
Zhang, Z.G. et al. A model of mini-embolic stroke offers measurements of the neurovascular unit response in the living mouse. Stroke 36, 2701–2704 (2005).
Zuo, Y., Lin, A., Chang, P. & Gan, W.B. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron 46, 181–189 (2005).
Zuo, Y., Yang, G., Kwon, E. & Gan, W.B. Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature 436, 261–265 (2005).
Kim, J.V., Kang, S.S., Dustin, M.L. & McGavern, D.B. Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis. Nature 457, 191–195 (2009).
Wake, H., Moorhouse, A.J., Jinno, S., Kohsaka, S. & Nabekura, J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J. Neurosci. 29, 3974–3980 (2009).
Haynes, S.E. et al. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat. Neurosci. 9, 1512–1519 (2006).
Denk, W., Strickler, J.H. & Webb, W.W. Two-photon laser scanning fluorescence microscopy. Science 248, 73–76 (1990).
Theer, P., Hasan, M.T. & Denk, W. Two-photon imaging to a depth of 1000 microm in living brains by use of a Ti:Al2O3 regenerative amplifier. Opt. Lett. 28, 1022–1024 (2003).
Wu, S.H. et al. Ankyrin repeat-rich membrane spanning/Kidins220 protein regulates dendritic branching and spine stability in vivo . Dev. Neurobiol. 69, 547–557 (2009).
Zhang, S. & Murphy, T.H. Imaging the impact of cortical microcirculation on synaptic structure and sensory-evoked hemodynamic responses in vivo . PLoS Biol. 5, e119 (2007).
Christie, R.H. et al. Growth arrest of individual senile plaques in a model of Alzheimer's disease observed by in vivo multiphoton microscopy. J. Neurosci. 21, 858–864 (2001).
Yan, P. et al. Characterizing the appearance and growth of amyloid plaques in APP/PS1 mice. J. Neurosci. 29, 10706–10714 (2009).
Masino, S.A., Kwon, M.C., Dory, Y. & Frostig, R.D. Characterization of functional organization within rat barrel cortex using intrinsic signal optical imaging through a thinned skull. Proc. Natl. Acad. Sci. USA 90, 9998–10002 (1993).
Helm, P.J., Ottersen, O.P. & Nase, G. Analysis of optical properties of the mouse cranium—implications for in vivo multi photon laser scanning microscopy. J. Neurosci. Methods 178, 316–322 (2009).
Holtmaat, A., Wilbrecht, L., Knott, G.W., Welker, E. & Svoboda, K. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature 441, 979–983 (2006).
Holtmaat, A.J. et al. Transient and persistent dendritic spines in the neocortex in vivo . Neuron 45, 279–291 (2005).
Svoboda, K., Denk, W., Kleinfeld, D. & Tank, D.W. In vivo dendritic calcium dynamics in neocortical pyramidal neurons. Nature 385, 161–165 (1997).
Trachtenberg, J.T. et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature 420, 788–794 (2002).
De Paola, V. et al. Cell type-specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron 49, 861–875 (2006).
Hofer, S.B., Mrsic-Flogel, T.D., Bonhoeffer, T. & Hubener, M. Experience leaves a lasting structural trace in cortical circuits. Nature 457, 313–317 (2009).
Keck, T. et al. Massive restructuring of neuronal circuits during functional reorganization of adult visual cortex. Nat. Neurosci. 11, 1162–1167 (2008).
Lee, W.C. et al. Dynamic remodeling of dendritic arbors in GABAergic interneurons of adult visual cortex. PLoS Biol. 4, e29 (2006).
Holtmaat, A. et al. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat. Protoc. 4, 1128–1144 (2009).
Bhatt, D.H., Zhang, S. & Gan, W.B. Dendritic spine dynamics. Annu. Rev. Physiol. 71, 261–282 (2009).
Meyer, M.P., Niell, C.M. & Smith, S.J. Brain imaging: how stable are synaptic connections? Curr. Biol. 13, R180–R182 (2003).
Ottersen, O.P. & Helm, P.J. How hardwired is the brain? Nature 420, 751–752 (2002).
Giepmans, B.N., Adams, S.R., Ellisman, M.H. & Tsien, R.Y. The fluorescent toolbox for assessing protein location and function. Science 312, 217–224 (2006).
Livet, J. et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 450, 56–62 (2007).
Feng, G. et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51 (2000).
Jung, S. et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell Biol. 20, 4106–4114 (2000).
Spires, T.L. et al. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J. Neurosci. 25, 7278–7287 (2005).
Acknowledgements
This work was supported by grants from the National Institutes of Health to W.-B.G. and J.G. as well as an Ellison Medical Foundation/AFAR Postdoctoral Fellowship to G.Y.
Author information
Authors and Affiliations
Contributions
All authors contributed to the development/improvement of the thinned-skull imaging technique and the manuscript preparation. G.Y. wrote the initial draft and made the figures. F.P. obtained the movie of imaging through a thinned-skull preparation. C.P. conducted microglia-imaging experiment. J.G. and W.G. were responsible for the initial development of the thinned-skull imaging approach. G.Y. and F.P. improved the technique.
Corresponding authors
Supplementary information
Supplementary Figure 1 | Home made head immobilization device.
Head immobilization device includes a custom built plate and a skull holder. The custom built plate was made of a 14×10×0.1 cm aluminum plate, two 18×18×18 mm aluminum blocks, two ¼ inch screws and two spacers. The skull holder was made of 3 to 4 double-edge shaving blades. All experiments using animals were carried out under institutional and national guidelines. (PDF 228 kb)
Supplementary Figure 2 | The dendrites and spines can be clearly visualized on a conventional fluorescence microscope after proper thinning of the skull.
(a-c) Images taken from a CCD camera equipped on a fluorescence microscope. Arrows indicate the spines located in the focal plane of the camera. Structures that were out of focus appear blurred in these images. Scale bar, 10 µm. All experiments using animals were carried out under institutional and national guidelines. (PDF 99 kb)
Supplementary Movie 1
Neuronal structures imaged at high resolution through a thinned-skull window in 1-month old YFP-H mice. The whole movie is an image stack of optical sections that span the thinned skull (∼20 µm in thickness), meningeal layers and neuronal structures within the first 100 µm under the pial surface. The imaged region is 66.6 µm × 66.6 µm and step size is 0.75 µm. The movie is played at a speed of 8 frames (6 µm) per second. Note there is a ∼20 µm space between the skull and the pial surface representing the subarachnoid space. This space is not well visualized when the skull is depressed during the thinning process. All experiments using animals were carried out under institutional and national guidelines. (MOV 3998 kb)
Rights and permissions
About this article
Cite this article
Yang, G., Pan, F., Parkhurst, C. et al. Thinned-skull cranial window technique for long-term imaging of the cortex in live mice. Nat Protoc 5, 201–208 (2010). https://doi.org/10.1038/nprot.2009.222
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2009.222
This article is cited by
-
EF1α-associated protein complexes affect dendritic spine plasticity by regulating microglial phagocytosis in Fmr1 knock-out mice
Molecular Psychiatry (2024)
-
Synchronized activity of sensory neurons initiates cortical synchrony in a model of neuropathic pain
Nature Communications (2023)
-
A sleep-active basalocortical pathway crucial for generation and maintenance of chronic pain
Nature Neuroscience (2023)
-
Construction and use of an adaptive optics two-photon microscope with direct wavefront sensing
Nature Protocols (2023)
-
Transparent neural interfaces: challenges and solutions of microengineered multimodal implants designed to measure intact neuronal populations using high-resolution electrophysiology and microscopy simultaneously
Microsystems & Nanoengineering (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.