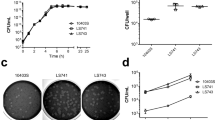
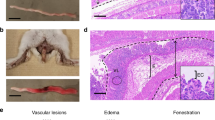
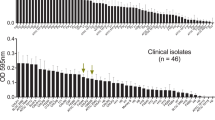
Abstract
Listeria monocytogenes causes listeriosis, a human foodborne infection leading to gastroenteritis, meningoencephalitis and maternofetal infections. InlA and InlB, two L. monocytogenes surface proteins, interact with their respective receptors E-cadherin and Met and mediate bacterial entry into human cultured cells. Here, we present protocols for studying listeriosis in three complementary animal models: (i) the human E-cadherin (hEcad) transgenic mouse line; (ii) the knock-in E16P mouse line; and (iii) the gerbil, in which both InlA–E-cadherin and InlB–Met species-specific interactions occur as in humans. Two routes of infection are described: oral inoculation, the natural route for infection; and intravenous inoculation that bypasses the intestinal barrier. We describe how to monitor L. monocytogenes infection, both qualitatively by imaging techniques and quantitatively by bacterial enumeration. The advantage of these methods over the classical intravenous inoculation of L. monocytogenes in wild-type mice (in which the InlA–E-cadherin interaction does not occur) is that it allows the pathophysiology of listeriosis to be studied in animal models relevant to humans, as they are permissive to the interactions that are thought to mediate L. monocytogenes crossing of human host barriers. The whole procedure (inoculation, in vivo imaging, bacterial enumeration, histopathology) takes one full week to complete, including 3 d of actual experiments.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Lecuit, M. Understanding how Listeria monocytogenes targets and crosses host barriers. Clin. Microbiol. Infect. 11, 430–436 (2005).
Hamon, M., Bierne, H. & Cossart, P. Listeria monocytogenes: a multifaceted model. Nat. Rev. Microbiol. 4, 423–434 (2006).
Murray, E.G.D., Webb, R.A. & Swann, M.B.R. A disease of rabbits characterised by a large mononuclear leukocytosis, caused by a hitherto undescribed bacillus Bacterium monocytogenes (n. sp.). J. Pathol. Bacteriol. 29, 407–439 (1926).
Pirie, J. A new disease of veld rodents, 'Tiger River Disease'. Pub. South Africa Inst. Med. Res. 62, 163–186 (1927).
Gill, D.A. Ovine bacterial encephalitis (circling disease) and the bacterial genus Listerella . Aust. Vet. J. 13, 46–56 (1937).
Lecuit, M. Human listeriosis and animal models. Microbes Infect. 9, 1216–1225 (2007).
Lecuit, M. et al. A single amino acid in E-cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes . EMBO J. 18, 3956–3963 (1999).
Mengaud, J., Ohayon, H., Gounon, P., Mege, R.M. & Cossart, P. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell 84, 923–932 (1996).
Shen, Y., Naujokas, M., Park, M. & Ireton, K. InIB-dependent internalization of Listeria is mediated by the Met receptor tyrosine kinase. Cell 103, 501–510 (2000).
Khelef, N., Lecuit, M., Bierne, H. & Cossart, P. Species specificity of the Listeria monocytogenes InlB protein. Cell Microbiol. 8, 457–470 (2006).
Disson, O. et al. Conjugated action of two species-specific invasion proteins for fetoplacental listeriosis. Nature 455, 1114–1118 (2008).
Gaillard, J.L., Berche, P. & Sansonetti, P. Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes . Infect. Immun. 52, 50–55 (1986).
Kocks, C. et al. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell 68, 521–531 (1992).
Pamer, E.G. Immune responses to Listeria monocytogenes . Nat. Rev. Immunol. 4, 812–823 (2004).
Lecuit, M. et al. A transgenic model for listeriosis: role of internalin in crossing the intestinal barrier. Science 292, 1722–1725 (2001).
Garner, M.R., Njaa, B.L., Wiedmann, M. & Boor, K.J. Sigma B contributes to Listeria monocytogenes gastrointestinal infection but not to systemic spread in the guinea pig infection model. Infect. Immun. 74, 876–886 (2006).
Cabanes, D., Lecuit, M. & Cossart, P. Animal models of listeria infection. Curr. Protoc. Microbiol. Chapter 9, Unit9B 1 (2008).
Tappe, J.P., Chandler, F.W., Westrom, W.K., Liu, S.K. & Dolensek, E.P. Listeriosis in seven bushy-tailed jirds. J. Am. Vet. Med. Assoc. 185, 1367–1370 (1984).
Contag, P.R. Bioluminescence imaging to evaluate infections and host response in vivo . Methods Mol. Biol. 415, 101–118 (2008).
Bron, P.A., Monk, I.R., Corr, S.C., Hill, C. & Gahan, C.G. Novel luciferase reporter system for in vitro and organ-specific monitoring of differential gene expression in Listeria monocytogenes . Appl. Environ. Microbiol. 72, 2876–2884 (2006).
Riedel, C.U. et al. Improved luciferase tagging system for Listeria monocytogenes allows real-time monitoring in vivo and in vitro . Appl. Environ. Microbiol. 73, 3091–3094 (2007).
Pasche, B. et al. Sex-dependent susceptibility to Listeria monocytogenes infection is mediated by differential interleukin-10 production. Infect. Immun. 73, 5952–5960 (2005).
Dramsi, S. et al. Entry of Listeria monocytogenes into hepatocytes requires expression of inIB, a surface protein of the internalin multigene family. Mol. Microbiol. 16, 251–261 (1995).
Lecuit, M., Ohayon, H., Braun, L., Mengaud, J. & Cossart, P. Internalin of Listeria monocytogenes with an intact leucine-rich repeat region is sufficient to promote internalization. Infect. Immun. 65, 5309–5319 (1997).
Dramsi, S., Levi, S., Triller, A. & Cossart, P. Entry of Listeria monocytogenes into neurons occurs by cell-to-cell spread: an in vitro study. Infect. Immun. 66, 4461–4468 (1998).
Perez-Garcia, C.C. et al. A simple procedure to perform intravenous injections in the Mongolian gerbil (Meriones unguiculatus). Lab. Anim. 37, 68–71 (2003).
Wollert, T. et al. Extending the host range of Listeria monocytogenes by rational protein design. Cell 129, 891–902 (2007).
Pautler, R.G. Mouse MRI: concepts and applications in physiology. Physiology (Bethesda) 19, 168–175 (2004).
Mansson, L.E. et al. Real-time studies of the progression of bacterial infections and immediate tissue responses in live animals. Cell Microbiol. 9, 413–424 (2007).
Monk, I.R., Casey, P.G., Cronin, M., Gahan, C.G. & Hill, C. Development of multiple strain competitive index assays for Listeria monocytogenes using pIMC; a new site-specific integrative vector. BMC Microbiol. 8, 96 (2008).
Acknowledgements
We thank E. Huillet, F. Langa, S. Vandormael-Pornin and C. Babinet for their respective contributions in the transgenesis and KI mouse projects; C. Hill for gift of the pPL2lux-PhlyA plasmid; M.-A. Nahori for her expertise in animal experiments; H. Khun for immunohistochemistry techniques; and V. Masse for help in setting up the competitive assay. M.L. thanks Olivier Lortholary for his support. Marc Lecuit and Pascale Cossart laboratories received financial support from the Institut Pasteur, Inserm, INRA, FRM and ANR.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Video 1: Intravenous infection of an anesthetized gerbil with L. monocytogenes
(see manuscript for details) (MOV 10588 kb)
Supplementary Video 2: Confocal 3D reconstruction of a gerbil placenta infected with EGD 42h pi
A 200 µm-thick section of a gerbil placenta was cut with a vibratome. The syncytiotrophoblast, placental capillaries and L. monocytogenes were stained in red (mouse anti-Ecad antibody), cyan (Isolectin B4) and green (rabbit anti-Listeria antibody), respectively. One hundred and fifty 200µmx200µm optical sections at 200 nm intervals were then imaged with a Leica TCS SP5 confocal microscope and a 3D-reconstruction was performed using the Imaris® software. (MOV 16375 kb)
Supplementary Video 3: Confocal 3D reconstruction of a gerbil placenta infected with EGDΔinlAB 42h pi
A 200 µm-thick section of a gerbil placenta was cut with a vibratome. The syncytiotrophoblast, placental capillaries and L. monocytogenes were stained in red (mouse anti-Ecad antibody), cyan (Isolectin B4) and green (rabbit anti-Listeria antibody), respectively. One hundred and fifty 250µmx250µm optical sections at 200 nm intervals were then imaged with a Leica TCS SP5 confocal microscope and a 3D-reconstruction was performed using the Imaris® software. (MOV 14209 kb)
Rights and permissions
About this article
Cite this article
Disson, O., Nikitas, G., Grayo, S. et al. Modeling human listeriosis in natural and genetically engineered animals. Nat Protoc 4, 799–810 (2009). https://doi.org/10.1038/nprot.2009.66
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2009.66
This article is cited by
-
Bacterial inhibition of Fas-mediated killing promotes neuroinvasion and persistence
Nature (2022)
-
Listeria monocytogenes faecal carriage is common and depends on the gut microbiota
Nature Communications (2021)
-
Hypervirulent Listeria monocytogenes clones’ adaption to mammalian gut accounts for their association with dairy products
Nature Communications (2019)
-
A hybrid sub-lineage of Listeria monocytogenes comprising hypervirulent isolates
Nature Communications (2019)
-
Uncovering Listeria monocytogenes hypervirulence by harnessing its biodiversity
Nature Genetics (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.