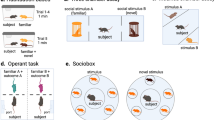
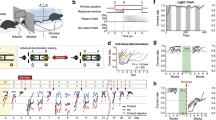
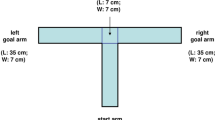
Abstract
Testing declarative memory in laboratory rodents can provide insights into the fundamental mechanisms underlying this type of learning and memory processing, and these insights are likely to be applicable to humans. Here we provide a detailed description of the social discrimination procedure used to investigate recognition memory in rats and mice, as established during the last 20 years in our laboratory. The test is based on the use of olfactory signals for social communication in rodents; this involves a direct encounter between conspecifics, during which the investigatory behavior of the experimental subject serves as an index for learning and memory performance. The procedure is inexpensive, fast and very reliable, but it requires well-trained human observers. We include recent modifications to the procedure that allow memory extinction to be investigated by retroactive and proactive interference, and that enable the dissociated analysis of the central nervous processing of the volatile fraction of an individual's olfactory signature. Depending on the memory retention interval under study (short-term memory, intermediate-term memory, long-term memory or long-lasting memory), the protocol takes ∼10 min or up to several days to complete.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Ebbinghaus, H. Über das Gedächtnis—Untersuchungen zur experimentellen Psychologie (Duncker & Humblot, Leipzig, 1885).
Schacter, D.L. Implicit memory: history and current status. J. Exp. Psychol.: Learning, Memory, and Cognition 13, 501–518 (1987).
Steckler, T., Drinkenburg, W.H., Sahgal, A. & Aggleton, J.P. Recognition memory in rats—I. Concepts and classification. Prog. Neurobiol. 54, 289–311 (1998).
Accolla, R. & Carleton, A. Internal body state influences topographical plasticity of sensory representations in the rat gustatory cortex. Proc. Natl. Acad. Sci. USA 105, 4010–4015 (2008).
McGaugh, J.L. Make mild moments memorable: add a little arousal. Trends Cogn. Sci. 10, 345–347 (2006).
Kamprath, K. & Wotjak, C.T. Nonassociative learning processes determine expression and extinction of conditioned fear in mice. Learn Mem. 11, 770–786 (2004).
Dantzer, R., Bluthe, R.M. & Kelley, K.W. Androgen-dependent vasopressinergic neurotransmission attenuates interleukin-1-induced sickness behavior. Brain Res. 557, 115–120 (1991).
Thor, D.H. & Holloway, W.R. Social memory of the male laboratory rat. J Comp. Physiol. Psychol. 96, 1000–1006 (1982).
Dantzer, R., Bluthe, R.M., Koob, G.F. & Le Moal, M. Modulation of social memory in male rats by neurohypophyseal peptides. Psychopharmacology (Berl.) 91, 363–368 (1987).
Engelmann, M., Wotjak, C.T. & Landgraf, R. Social discrimination procedure: an alternative method to investigate juvenile recognition abilities in rats. Physiol. Behav. 58, 315–321 (1995).
Reijmers, L.G., Leus, I.E., Burbach, J.P., Spruijt, B.M. & van Ree, J.M. Social memory in the rat: circadian variation and effect of circadian rhythm disruption. Physiol. Behav. 72, 305–309 (2001).
Sterlemann, V. et al. Chronic social stress during adolescence induces cognitive impairment in aged mice. Hippocampus 20, 540–549 (2010).
Choleris, E. et al. Involvement of estrogen receptor alpha, beta and oxytocin in social discrimination: a detailed behavioral analysis with knockout female mice. Genes Brain Behav. 5, 528–539 (2006).
Macbeth, A.H., Edds, J.S. & Young, W.S. III Housing conditions and stimulus females: a robust social discrimination task for studying male rodent social recognition. Nat. Protoc. 4, 1574–1581 (2009).
Bluthe, R.M. & Dantzer, R. Role of the vomeronasal system in vasopressinergic modulation of social recognition in rats. Brain Res. 604, 205–210 (1993).
Noack, J. et al. Different importance of the volatile and non-volatile fractions of an olfactory signature for individual social recognition in rats versus mice and short-term versus long-term memory. Neurobiol. Learn. Mem. 94, 568–575 (2010).
Popik, P., Vetulani, J., Bisaga, A. & van Ree, J.M. Recognition cue in the rat's social memory paradigm. J. Basic Clin. Physiol. Pharmacol. 2, 315–327 (1991).
Page, D.T., Kuti, O.J. & Sur, M. Computerized assessment of social approach behavior in mouse. Front Behav. Neurosci. 3, 48 (2009).
Insel, T.R. & Fernald, R.D. How the brain processes social information: searching for the social brain. Annu. Rev. Neurosci. 27, 697–722 (2004).
Engelmann, M. Competition between two memory traces for long-term recognition memory. Neurobiol. Learn. Mem. 91, 58–65 (2009).
Squires, A.S., Peddle, R., Milway, S.J. & Harley, C.W. Cytotoxic lesions of the hippocampus do not impair social recognition memory in socially housed rats. Neurobiol. Learn. Mem. 85, 95–101 (2006).
Richter, K., Wolf, G. & Engelmann, M. Social recognition memory requires two stages of protein synthesis in mice. Learn. Mem. 12, 407–413 (2005).
Wanisch, K., Wotjak, C.T. & Engelmann, M. Long-lasting second stage of recognition memory consolidation in mice. Behavioural. Brain Res. 186, 191–196 (2008).
Dluzen, D.E., Muraoka, S., Engelmann, M., Ebner, K. & Landgraf, R. Oxytocin induces preservation of social recognition in male rats by activating alpha-adrenoceptors of the olfactory bulb. Eur. J. Neurosci. 12, 760–766 (2000).
Dluzen, D.E., Muraoka, S., Engelmann, M. & Landgraf, R. The effects of infusion of arginine vasopressin, oxytocin, or their antagonists into the olfactory bulb upon social recognition responses in male rats. Peptides 19, 999–1005 (1998).
Landgraf, R. et al. V1 vasopressin receptor antisense oligodeoxynucleotide into septum reduces vasopressin binding, social discrimination abilities, and anxiety-related behavior in rats. J. Neurosci. 15, 4250–4258 (1995).
Liebsch, G., Landgraf, R., Engelmann, M., Lorscher, P. & Holsboer, F. Differential behavioural effects of chronic infusion of CRH 1 and CRH 2 receptor antisense oligonucleotides into the rat brain. J. Psychiatr. Res. 33, 153–163 (1999).
Tobin, V.A. et al. An intrinsic vasopressin system in the olfactory bulb is involved in social recognition. Nature 464, 413–417 (2010).
Jüch, M. et al. Congenital lack of nNOS impairs long-term social recognition memory and alters the olfactory bulb proteome. Neurobiol. Learn. Mem. 92, 469–484 (2009).
Kogan, J.H., Frankland, P.W. & Silva, A.J. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus 10, 47–56 (2000).
Bluthe, R.M. & Dantzer, R. Social recognition does not involve vasopressinergic neurotransmission in female rats. Brain Res. 535, 301–304 (1990).
Bluthe, R.M., Schoenen, J. & Dantzer, R. Androgen-dependent vasopressinergic neurons are involved in social recognition in rats. Brain Res. 519, 150–157 (1990).
Engelmann, M., Ebner, K., Wotjak, C.T. & Landgraf, R. Endogenous oxytocin is involved in short-term olfactory memory in female rats. Behav. Brain Res. 90, 89–94 (1998).
Engelmann, M. & Landgraf, R. Microdialysis administration of vasopressin into the septum improves social recognition in Brattleboro rats. Physiol. Behav. 55, 145–149 (1994).
Sanchez-Andrade, G. & Kendrick, K.M. Roles of alpha- and beta-estrogen receptors in mouse social recognition memory: effects of gender and the estrous cycle. Horm. Behav. 59, 114–122 (2011).
Terranova, J.P., Perio, A., Worms, P., Le Fur, G. & Soubrie, P. Social olfactory recognition in rodents: deterioration with age, cerebral ischaemia and septal lesion. Behav. Pharmacol. 5, 90–98 (1994).
Prediger, R.D., Batista, L.C. & Takahashi, R.N. Caffeine reverses age-related deficits in olfactory discrimination and social recognition memory in rats. Involvement of adenosine A1 and A2A receptors. Neurobiol. Aging 26, 957–964 (2005).
Bothe, G.W.M., Bolivar, V.J., Vedder, M.J. & Geistfeld, J.G. Behavioral differences among fourteen inbred mouse strains commonly used as disease models. Comparative Med. 55, 326–334 (2005).
Camp, M. et al. Impaired Pavlovian fear extinction is a common phenotype across genetic lineages of the 129 inbred mouse strain. Genes, Brain and Behav. 8, 744–752 (2009).
Cook, M.N., Bolivar, V.J., McFadyen, M.P. & Flaherty, L. Behavioral differences among 129 substrains: implications for knockout and transgenic mice. Behav. Neurosci. 116, 600–611 (2002).
Hefner, K. et al. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J. Neurosci. 28, 8074–8085 (2008).
Owen, E.H., Logue, S.F., Rasmussen, D.L. & Wehner, J.M. Assessment of learning by the Morris water task and fear conditioning in inbred mouse strains and F1 hybrids: implications of genetic background for single gene mutations and quantitative trait loci analyses. Neuroscience 80, 1087–1099 (1997).
Simpson, E.M. et al. Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nat. Genet. 16, 19–27 (1997).
Thor, D.H. Testosterone and persistence of social investigation in laboratory rats. J. Comp. Physiol. Psychol. 94, 970–976 (1980).
Bluthe, R.M., Gheusi, G. & Dantzer, R. Gonadal steroids influence the involvement of arginine vasopressin in social recognition in mice. Psychoneuroendocrinology 18, 323–335 (1993).
Prediger, R.D., Fernandes, D. & Takahashi, R.N. Blockade of adenosine A2A receptors reverses short-term social memory impairments in spontaneously hypertensive rats. Behav. Brain Res. 159, 197–205 (2005).
Müller, G.E. & Pilzecker, A. Experimentelle Beiträge zur Lehre vom Gedächtnis. Zeitschrift für Psychologie und Physiologie der Sinnesorgane EB 1–300 (1900).
Burman, O.H. & Mendl, M. Short-term social memory in the laboratory rat: its susceptibility to disturbance. Appl. Anim. Behav. Sci. 67, 241–254 (2000).
Amano, K. et al. Estimation of the timing of human visual perception from magnetoencephalography. J. Neurosci. 26, 3981–3991 (2006).
Welker, W.I. Analysis of sniffing of the albino rat. Behaviour 22, 223–244 (1964).
Wesson, D.W., Donahou, T.N., Johnson, M.O. & Wachowiak, M. Sniffing behavior of mice during performance in odor-guided tasks. Chem. Senses 33, 581–596 (2008).
Kimchi, T., Xu, J. & Dulac, C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature 448, 1009–1014 (2007).
Perio, A. et al. Specific modulation of social memory in rats by cholinomimetic and nootropic drugs, by benzodiazepine inverse agonists, but not by psychostimulants. Psychopharmacology (Berl.) 97, 262–268 (1989).
Acknowledgements
We thank R. Murau and M. Radscheidt for expert support in behavioral testing. We also thank G. Leng, Edinburgh, for polishing the English. During the writing of this paper, the authors were supported by the Deutsche Forschungsgemeinschaft (EN 366/6-1 and EN 366/8-1).
Author information
Authors and Affiliations
Contributions
M.E. and J.N. designed the study, J.H. performed some of the behavioral experiments, and M.E. and J.N. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
No impact of female sex cycle on long-term social discrimination. The same group-housed female mice were tested for their social recognition abilities under estrous and non-estrous conditions at an retention interval of 24 h. The stage of the cycle was determined using microscopical analysis of vaginal smears taken ∼ 1 h after choice. Statistical analysis shows that experimental subjects successfully discriminated between the stimulus animals independently upon cyclus stage during choice. Statistics was performed using paired t-test. (JPG 329 kb)
Supplementary Fig. 2
Social discrimination is sensitive to interference phenomena. Examples showing schematically the experimental design (insets) and representative results obtained with same group-housed adult male mice as experimental subjects without (A) and with experimental induction of interference (B,C) for long-term social recognition memory. A, demonstrates intact recognition memory performance of these animals in the standard social discrimination protocol. B, Presentation of an interference juvenile (hatched) during the retention interval of 24h (6h after sampling) impairs recognition of the familiar juvenile. C, Presentation of an interference juvenile (hatched) 3h before sampling impairs recognition of the familiar juvenile at the retention interval of 21 h. Statistics was performed using paired t-test. Please note that the effect of interference is time-dependent. (JPG 533 kb)
Supplementary Fig. 3
Stimulus animal labelling. Schematic drawing showing the relative position and length of the marks used for labelling at the dorsal side of a juvenile. (JPG 165 kb)
Rights and permissions
About this article
Cite this article
Engelmann, M., Hädicke, J. & Noack, J. Testing declarative memory in laboratory rats and mice using the nonconditioned social discrimination procedure. Nat Protoc 6, 1152–1162 (2011). https://doi.org/10.1038/nprot.2011.353
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2011.353
This article is cited by
-
Detection, processing and reinforcement of social cues: regulation by the oxytocin system
Nature Reviews Neuroscience (2023)
-
A quantitative analysis of spontaneous alternation behaviors on a Y-maze reveals adverse effects of acute social isolation on spatial working memory
Scientific Reports (2023)
-
Dynamic and stable hippocampal representations of social identity and reward expectation support associative social memory in male mice
Nature Communications (2023)
-
The extracellular matrix and perineuronal nets in memory
Molecular Psychiatry (2022)
-
Top-down acetylcholine signaling via olfactory bulb vasopressin cells contributes to social discrimination in rats
Communications Biology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.